Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials
- PMID: 29272162
- PMCID: PMC5815402
- DOI: 10.1200/JCO.2017.75.2998
Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: A Comparison With Prostate-Specific Antigen Across Five Randomized Phase III Clinical Trials
Abstract
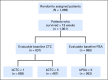
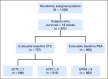
Purpose Measures of response that are clinically meaningful and occur early are an unmet need in metastatic castration-resistant prostate cancer clinical research and practice. We explored, using individual patient data, week 13 circulating tumor cell (CTC) and prostate-specific antigen (PSA) response end points in five prospective randomized phase III trials that enrolled a total of 6,081 patients-COU-AA-301, AFFIRM, ELM-PC-5, ELM-PC-4, and COMET-1- ClinicalTrials.Gov identifiers: NCT00638690, NCT00974311, NCT01193257, NCT01193244, and NCT01605227, respectively. Methods Eight response end points were explored. CTC nonzero at baseline and 0 at 13 weeks (CTC0); CTC conversion (≥ 5 CTCs at baseline, ≤ 4 at 13 weeks-the US Food and Drug Administration cleared response measure); a 30%, 50%, and 70% decrease in CTC count; and a 30%, 50%, and 70% decrease in PSA level. Patients missing week-13 values were considered nonresponders. The discriminatory strength of each end point with respect to overall survival in each trial was assessed using the weighted c-index. Results Of the eight response end points, CTC0 and CTC conversion had the highest weighted c-indices, with smaller standard deviations. For CTC0, the mean (standard deviation) was 0.81 (0.04); for CTC conversion, 0.79 (0.03); for 30% decrease in CTC count, 0.72 (0.06); for 50% decrease in CTC count, 0.72 (0.06); for 70% decrease in CTC count, 0.73 (0.05); for 30% decrease in PSA level, 0.71 (0.03); for 50% decrease in PSA level, 0.72 (0.06); and for 70% decrease in PSA level, 0.74 (0.05). Seventy-five percent of eligible patients could be evaluated with the CTC0 end point, compared with 51% with the CTC conversion end point. Conclusion The CTC0 and CTC conversion end points had the highest discriminatory power for overall survival. Both are robust and meaningful response end points for early-phase metastatic castration-resistant prostate cancer clinical trials. CTC0 is applicable to a significantly higher percentage of patients than CTC conversion.
Figures






Comment in
-
Circulating Tumor Cell Eradication as an Intermediate Efficacy End Point for Metastatic Castration-Resistant Prostate Cancer: Is There Enough Evidence?J Clin Oncol. 2018 Feb 20;36(6):525-527. doi: 10.1200/JCO.2017.76.5487. Epub 2017 Dec 22. J Clin Oncol. 2018. PMID: 29272163 No abstract available.
-
Reply to C. Ren et al.J Clin Oncol. 2018 Aug 1;36(22):2354-2356. doi: 10.1200/JCO.2018.78.2672. Epub 2018 Jun 12. J Clin Oncol. 2018. PMID: 29894273 No abstract available.
-
Circulating Tumor Cell Number as a Response Measure of Prolonged Survival for Metastatic Castration-Resistant Prostate Cancer: Is Real Circulating Tumor Cell Number Superior to Prostate-Specific Antigen?J Clin Oncol. 2018 Aug 1;36(22):2353-2354. doi: 10.1200/JCO.2018.78.1401. Epub 2018 Jun 12. J Clin Oncol. 2018. PMID: 29894275 No abstract available.
-
Reply to C. Ren et al.J Clin Oncol. 2018 Aug 1;36(22):2356-2357. doi: 10.1200/JCO.2018.78.2680. Epub 2018 Jun 12. J Clin Oncol. 2018. PMID: 29894276 No abstract available.
References
-
- Paoletti C, Hayes DF: Circulating tumor cells. Adv Exp Med Biol 882:235-258, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

