Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(OH)2D3
- PMID: 29272316
- PMCID: PMC5741250
- DOI: 10.1371/journal.pone.0190131
Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(OH)2D3
Abstract
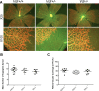
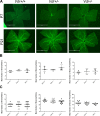
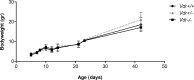
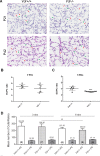
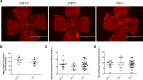
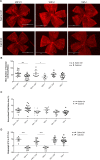
Vitamin D provides a significant benefit to human health, and its deficiency has been linked to a variety of diseases including cancer. Vitamin D exhibits anticancer effects perhaps through inhibition of angiogenesis. We previously showed that the active form of vitamin D (1, 25(OH)2D3; calcitriol) is a potent inhibitor of angiogenesis in mouse model of oxygen-induced ischemic retinopathy (OIR). Many of vitamin D's actions are mediated through vitamin D receptor (VDR). However, the role VDR expression plays in vascular development and inhibition of neovascularization by 1, 25(OH)2D3 remains unknown. Here using wild type (Vdr +/+) and Vdr-deficient (Vdr -/-) mice, we determined the impact of Vdr expression on postnatal development of retinal vasculature and retinal neovascularization during OIR. We observed no significant effect on postnatal retinal vascular development in Vdr -/- mice up to postnatal day 21 (P21) compared with Vdr +/+ mice. However, we observed an increase in density of pericytes (PC) and a decrease in density of endothelial cells (EC) in P42 Vdr -/- mice compared with Vdr +/+ mice, resulting in a significant decrease in the EC/PC ratio. Although we observed no significant impact on vessel obliteration and retinal neovascularization in Vdr -/- mice compared with Vdr +/+ mice during OIR, the VDR expression was essential for inhibition of retinal neovascularization by 1, 25(OH)2D3. In addition, the adverse impact of 1, 25(OH)2D3 treatment on the mouse bodyweight was also dependent on VDR expression. Thus, VDR expression plays a significant role during retinal vascular development, especially during maturation of retinal vasculature by promoting PC quiescence and EC survival, and inhibition of ischemia-mediated retinal neovascularization by 1, 25(OH)2D3.
Conflict of interest statement
Figures
References
-
- Zittermann A. Vitamin D-Cholecalciferol In: Herrmann W, Obeid R, editors. Vitamins in the Prevention of Human Diseases. Germany: De Gruyter; 2011. p. 363–95.
-
- Jamali N, Sorenson C, Sheibani N. Vitamin D attenuates proangiogenic properties of pericytes through increased production of VEGF (679.1). The FASEB Journal. 2014;28(1 Supplement).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

