Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration
- PMID: 29272357
- PMCID: PMC5837493
- DOI: 10.1093/brain/awx339
Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration
Abstract
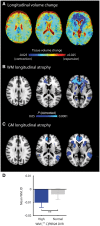
Survivors of a traumatic brain injury can deteriorate years later, developing brain atrophy and dementia. Traumatic brain injury triggers chronic microglial activation, but it is unclear whether this is harmful or beneficial. A successful chronic-phase treatment for traumatic brain injury might be to target microglia. In experimental models, the antibiotic minocycline inhibits microglial activation. We investigated the effect of minocycline on microglial activation and neurodegeneration using PET, MRI, and measurement of the axonal protein neurofilament light in plasma. Microglial activation was assessed using 11C-PBR28 PET. The relationships of microglial activation to measures of brain injury, and the effects of minocycline on disease progression, were assessed using structural and diffusion MRI, plasma neurofilament light, and cognitive assessment. Fifteen patients at least 6 months after a moderate-to-severe traumatic brain injury received either minocycline 100 mg orally twice daily or no drug, for 12 weeks. At baseline, 11C-PBR28 binding in patients was increased compared to controls in cerebral white matter and thalamus, and plasma neurofilament light levels were elevated. MRI measures of white matter damage were highest in areas of greater 11C-PBR28 binding. Minocycline reduced 11C-PBR28 binding (mean Δwhite matter binding = -23.30%, 95% confidence interval -40.9 to -5.64%, P = 0.018), but increased plasma neurofilament light levels. Faster rates of brain atrophy were found in patients with higher baseline neurofilament light levels. In this experimental medicine study, minocycline after traumatic brain injury reduced chronic microglial activation while increasing a marker of neurodegeneration. These findings suggest that microglial activation has a reparative effect in the chronic phase of traumatic brain injury.
Keywords: microglia; minocycline; neurodegeneration; positron emission tomography; traumatic brain injury.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Similar articles
-
Diffuse axonal injury predicts neurodegeneration after moderate-severe traumatic brain injury.Brain. 2020 Dec 1;143(12):3685-3698. doi: 10.1093/brain/awaa316. Brain. 2020. PMID: 33099608
-
Differential effects of minocycline on microglial activation and neurodegeneration following closed head injury in the neonate rat.Exp Neurol. 2017 Apr;290:1-14. doi: 10.1016/j.expneurol.2016.12.010. Epub 2016 Dec 27. Exp Neurol. 2017. PMID: 28038986 Free PMC article.
-
Minocycline Transiently Reduces Microglia/Macrophage Activation but Exacerbates Cognitive Deficits Following Repetitive Traumatic Brain Injury in the Neonatal Rat.J Neuropathol Exp Neurol. 2016 Mar;75(3):214-26. doi: 10.1093/jnen/nlv021. Epub 2016 Jan 29. J Neuropathol Exp Neurol. 2016. PMID: 26825312 Free PMC article.
-
Traumatic brain injuries.Nat Rev Dis Primers. 2016 Nov 17;2:16084. doi: 10.1038/nrdp.2016.84. Nat Rev Dis Primers. 2016. PMID: 27853132 Review.
-
Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia.J Neurol Neurosurg Psychiatry. 2019 Nov;90(11):1221-1233. doi: 10.1136/jnnp-2017-317557. Epub 2019 Sep 21. J Neurol Neurosurg Psychiatry. 2019. PMID: 31542723 Free PMC article. Review.
Cited by
-
Complex Autoantibody Responses Occur following Moderate to Severe Traumatic Brain Injury.J Immunol. 2021 Jul 1;207(1):90-100. doi: 10.4049/jimmunol.2001309. Epub 2021 Jun 18. J Immunol. 2021. PMID: 34145056 Free PMC article.
-
Persistent Neurovascular Unit Dysfunction: Pathophysiological Substrate and Trigger for Late-Onset Neurodegeneration After Traumatic Brain Injury.Front Neurosci. 2020 Jun 9;14:581. doi: 10.3389/fnins.2020.00581. eCollection 2020. Front Neurosci. 2020. PMID: 32581697 Free PMC article.
-
Effects of Minocycline on Neurological Outcomes In Patients With Acute Traumatic Brain Injury: A Pilot Study.Iran J Pharm Res. 2019 Spring;18(2):1086-1096. doi: 10.22037/ijpr.2019.1100677. Iran J Pharm Res. 2019. PMID: 31531090 Free PMC article.
-
Neuroprotection in Traumatic Brain Injury: Mesenchymal Stromal Cells can Potentially Overcome Some Limitations of Previous Clinical Trials.Front Neurol. 2018 Oct 24;9:885. doi: 10.3389/fneur.2018.00885. eCollection 2018. Front Neurol. 2018. PMID: 30405517 Free PMC article. Review.
-
The synergistic effect of minocycline and azole antifungal drugs against Scedosporium and Lomentospora species.BMC Microbiol. 2022 Jan 12;22(1):21. doi: 10.1186/s12866-021-02433-6. BMC Microbiol. 2022. PMID: 35016611 Free PMC article.
References
-
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med 1998; 338: 20–4. - PubMed
-
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. - PubMed
-
- Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 2016a; 91: 494–6. - PubMed
-
- Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, et al. Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron 2016b; 91: 1–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

