Human Immunodeficiency Virus Antiretroviral Resistance and Transmission in Mother-Infant Pairs Enrolled in a Large Perinatal Study
- PMID: 29272365
- PMCID: PMC5961106
- DOI: 10.1093/cid/cix1104
Human Immunodeficiency Virus Antiretroviral Resistance and Transmission in Mother-Infant Pairs Enrolled in a Large Perinatal Study
Abstract
Background: The presence of antiretroviral drug-associated resistance mutations (DRMs) may be particularly problematic in human immunodeficiency virus (HIV)-infected pregnant women as it can lead to mother-to-child transmission (MTCT) of resistant HIV strains. This study evaluated the prevalence and the effect of antiretroviral DRMs in previously untreated mother-infant pairs.
Methods: A case-control design of 1:4 (1 transmitter to 4 nontransmitters) was utilized to evaluate DRMs as a predictor of HIV MTCT in specimens obtained from mother-infant pairs. ViroSeq HIV-1 genotyping was performed on mother-infant specimens to assess for clinically relevant DRMs.
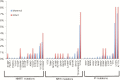
Results: One hundred forty infants acquired HIV infection; of these, 123 mother-infant pairs (88%) had specimens successfully amplified using ViroSeq and assessed for drug resistance genotyping. Additionally, 483 of 560 (86%) women who did not transmit HIV to infants also had samples evaluated for DRMs. Sixty-three of 606 (10%) women had clinically relevant DRMs; 12 (2%) had DRMs against >1 drug class. Among 123 HIV-infected infants, 13 (11%) had clinically relevant DRMs, with 3 (2%) harboring DRMs against >1 drug class. In univariate and multivariate analyses, DRMs in mothers were not associated with increased HIV MTCT (adjusted odds ratio, 0.8 [95% confidence interval, .4-1.5]). Presence of DRMs in transmitting mothers was strongly associated with DRM presence in their infants (P < .001).
Conclusions: Preexisting DRMs were common in untreated HIV-infected pregnant women, but did not increase the risk of HIV MTCT. However, if women with DRMs are not virologically suppressed, they may transmit resistant mutations, thus complicating infant management.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive Available at: http://www.unaids.org/en/resources/documents/2015/JC2774_2015ProgressRep.... Accessed 13 February 2017.
-
- Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev 2011; 7:CD003510. - PubMed
-
- Arrivé E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol 2007; 36:1009–21. - PubMed
-
- Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med 2007; 356:135–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

