Monitoring adherence to guidelines of antibiotic use in pediatric pneumonia: the MAREA study
- PMID: 29273072
- PMCID: PMC5741879
- DOI: 10.1186/s13052-017-0432-2
Monitoring adherence to guidelines of antibiotic use in pediatric pneumonia: the MAREA study
Abstract
Background: Children are the most vulnerable population exposed to the use of antibiotics often incorrectly prescribed for the treatment of infections really due to viruses rather than to bacteria. We designed the MAREA study which consisted of two different studies: i) a surveillance study to monitor the safety/efficacy of the antibiotics for the treatment of pneumonia (CAP), pharyngotonsillitis and acute otitis media in children younger than 14 yrs old, living in Liguria, North-West Italy and ii) a pre-/post-interventional study to evaluate the appropriateness of antibiotic prescription for the treatment these infections. In this paper, we show only results of the appropriateness study about the antibiotic prescription for the treatment of pneumonia.
Methods: Patients included in this study met the following inclusion criteria: i) admission to the Emergency/Inpatient Dpt/outpatient clinic of primary care pediatricians for pneumonia requiring antibiotics, ii) informed written consent. The practice of prescribing antibiotics was evaluated before-and-after a 1 day-educational intervention on International/National recommendations.
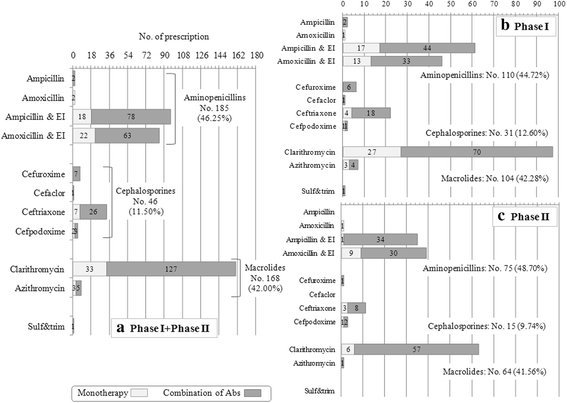
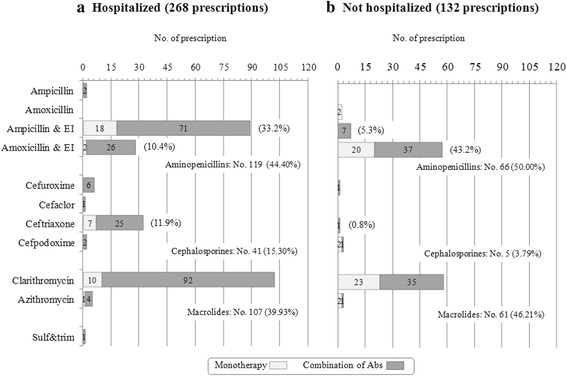
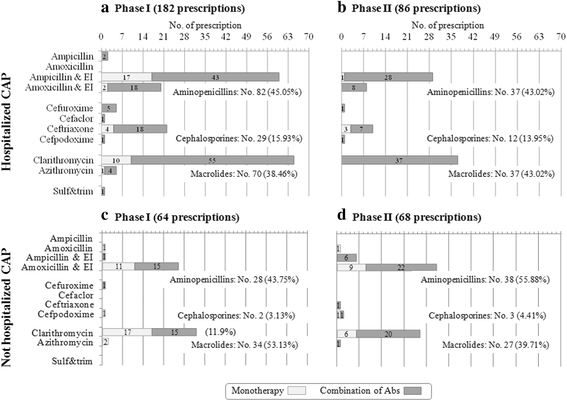
Results: Global adherence to guidelines was fulfilled in 45%: main reason for discordance was duration (shorter than recommended). Macrolide monotherapy and cephalosporins were highly prescribed; ampicillin/amoxicillin use was limited. 61% of patients received >1 antibiotic; parenteral route was used in 33%. After intervention, i) in all CAP, cephalosporin prescription decreased (-23%) and the inappropriate macrolide prescriptions was halved and, ii) in not hospitalized CAP (notH-CAP), macrolides were prescribed less frequently (-25%) and global adherence to guidelines improved (+39%); and iii) in H-CAP antibiotic choice appropriateness increase.
Conclusion: Prescribing practices were sufficiently appropriate but widespread preference for multidrug empirical regimens or macrolide in monotherapy deserve closer investigation.
Keywords: Antibiotic therapy; Appropriateness; Children; Pneumonia.
Conflict of interest statement
Ethics approval and consent to participate
The surveillance and the appropriateness studies were approved by Ethics Committee of the Gaslini Institute (PdP_SR_IGG001). For the surveillance study, all participants and their parents or tutors were informed in detail on the experimental procedure and the main scope of the study and provided written informed consent: For the appropriateness study, in the pre-intervention phase, pediatricians were kept unaware of the aim of the study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- NICE NICE Short Clinical Guidelines Technical Team, 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

