Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells
- PMID: 29273086
- PMCID: PMC5741939
- DOI: 10.1186/s13287-017-0742-8
Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells
Abstract
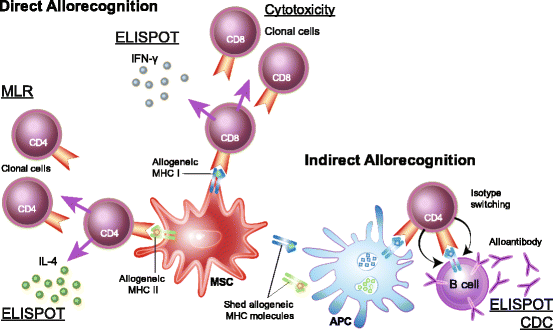
Background: Autologous and allogeneic adult mesenchymal stem/stromal cells (MSCs) are increasingly being investigated for treating a wide range of clinical diseases. Allogeneic MSCs are especially attractive due to their potential to provide immediate care at the time of tissue injury or disease diagnosis. The prevailing dogma has been that allogeneic MSCs are immune privileged, but there have been very few studies that control for matched or mismatched major histocompatibility complex (MHC) molecule expression and that examine immunogenicity in vivo. Studies that control for MHC expression have reported both cell-mediated and humoral immune responses to MHC-mismatched MSCs. The clinical implications of immune responses to MHC-mismatched MSCs are still unknown. Pre-clinical and clinical studies that document the MHC haplotype of donors and recipients and measure immune responses following MSC treatment are necessary to answer this critical question.
Conclusions: This review details what is currently known about the immunogenicity of allogeneic MSCs and suggests contemporary assays that could be utilized in future studies to appropriately identify and measure immune responses to MHC-mismatched MSCs.
Keywords: Allogeneic; Cytotoxicity; ELISPOT; Immunogenicity; Major histocompatibility complex; Mesenchymal stem cell; Microcytotoxicity; Mixed leukocyte reaction.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

