Pathological histone acetylation in Parkinson's disease: Neuroprotection and inhibition of microglial activation through SIRT 2 inhibition
- PMID: 29273397
- PMCID: PMC5821898
- DOI: 10.1016/j.neulet.2017.12.037
Pathological histone acetylation in Parkinson's disease: Neuroprotection and inhibition of microglial activation through SIRT 2 inhibition
Abstract
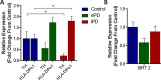
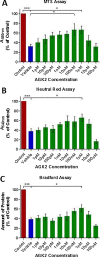
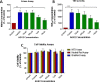
Parkinson's disease (PD) is associated with degeneration of nigrostriatal neurons due to intracytoplasmic inclusions composed predominantly of a synaptic protein called α-synuclein. Accumulations of α-synuclein are thought to 'mask' acetylation sites on histone proteins, inhibiting the action of histone acetyltransferase (HAT) enzymes in their equilibrium with histone deacetylases (HDACs), thus deregulating the dynamic control of gene transcription. It is therefore hypothesised that the misbalance in the actions of HATs/HDACs in neurodegeneration can be rectified with the use of HDAC inhibitors, limiting the deregulation of transcription and aiding neuronal homeostasis and neuroprotection in disorders such as PD. Here we quantify histone acetylation in the Substantia Nigra pars compacta (SNpc) in the brains of control, early and late stage PD cases to determine if histone acetylation is a function of disease progression. PD development is associated with Braak-dependent increases in histone acetylation. Concurrently, we show that as expected disease progression is associated with reduced markers of dopaminergic neurons and increased markers of activated microglia. We go on to demonstrate that in vitro, degenerating dopaminergic neurons exhibit histone hypoacetylation whereas activated microglia exhibit histone hyperacetylation. This suggests that the disease-dependent increase in histone acetylation observed in human PD cases is likely a combination of the contributions of both degenerating dopaminergic neurons and infiltrating activated microglia. The HDAC SIRT 2 has become increasingly implicated as a novel target for mediation of neuroprotection in PD: the neuronal and microglial specific effects of its inhibition however remain unclear. We demonstrate that SIRT 2 expression in the SNpc of PD brains remains relatively unchanged from controls and that SIRT 2 inhibition, via AGK2 treatment of neuronal and microglial cultures, results in neuroprotection of dopaminergic neurons and reduced activation of microglial cells. Taken together, here we demonstrate that histone acetylation is disease-dependently altered in PD, likely due the effects of dopaminergic neurodegeneration and microglial infiltration; yet SIRT 2 remains relatively unaltered with disease. Given the stable nature of SIRT 2 expression with disease and the effects of SIRT 2 inhibitor treatment on degenerating dopaminergic neurons and activated microglia detected in vitro, SIRT 2 inhibitors warrant further investigation as potential therapeutics for the treatment of the PD.
Keywords: Histone deacetylase inhibitor; Microglia; Neurodegeneration; Neuroprotection; Parkinson’s disease; SIRT 2.
Copyright © 2017 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease?Pharmacol Ther. 2013 Oct;140(1):34-52. doi: 10.1016/j.pharmthera.2013.05.010. Epub 2013 May 24. Pharmacol Ther. 2013. PMID: 23711791 Review.
-
T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an α-synuclein rat model of Parkinson's disease.J Neuroinflammation. 2020 Aug 15;17(1):242. doi: 10.1186/s12974-020-01911-4. J Neuroinflammation. 2020. PMID: 32799878 Free PMC article.
-
The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson's disease.J Neurochem. 2019 Jan;148(1):136-156. doi: 10.1111/jnc.14599. Epub 2018 Nov 26. J Neurochem. 2019. PMID: 30269333 Free PMC article.
-
Regulation of Histone Acetylation by Autophagy in Parkinson Disease.J Biol Chem. 2016 Feb 12;291(7):3531-40. doi: 10.1074/jbc.M115.675488. Epub 2015 Dec 23. J Biol Chem. 2016. PMID: 26699403 Free PMC article.
-
Pathological α-synuclein exacerbates the progression of Parkinson's disease through microglial activation.Toxicol Lett. 2017 Jan 4;265:30-37. doi: 10.1016/j.toxlet.2016.11.002. Epub 2016 Nov 16. Toxicol Lett. 2017. PMID: 27865851 Review.
Cited by
-
Epidrugs in the Therapy of Central Nervous System Disorders: A Way to Drive on?Cells. 2023 May 24;12(11):1464. doi: 10.3390/cells12111464. Cells. 2023. PMID: 37296584 Free PMC article. Review.
-
Epigenetic Changes in Prion and Prion-like Neurodegenerative Diseases: Recent Advances, Potential as Biomarkers, and Future Perspectives.Int J Mol Sci. 2022 Oct 20;23(20):12609. doi: 10.3390/ijms232012609. Int J Mol Sci. 2022. PMID: 36293477 Free PMC article. Review.
-
Current Advancement of Immunomodulatory Drugs as Potential Pharmacotherapies for Autoimmunity Based Neurological Diseases.Pharmaceuticals (Basel). 2022 Aug 29;15(9):1077. doi: 10.3390/ph15091077. Pharmaceuticals (Basel). 2022. PMID: 36145298 Free PMC article. Review.
-
Expression of clock gene Dbp in omental and mesenteric adipose tissue in patients with type 2 diabetes.BMJ Open Diabetes Res Care. 2020 Aug;8(1):e001465. doi: 10.1136/bmjdrc-2020-001465. BMJ Open Diabetes Res Care. 2020. PMID: 32816832 Free PMC article.
-
Regulation of Social Stress and Neural Degeneration by Activity-Regulated Genes and Epigenetic Mechanisms in Dopaminergic Neurons.Mol Neurobiol. 2020 Nov;57(11):4500-4510. doi: 10.1007/s12035-020-02037-7. Epub 2020 Aug 3. Mol Neurobiol. 2020. PMID: 32748368 Free PMC article. Review.
References
-
- Adams F., Rosa F., Kumar S., Edwards-Prasad J., Kentroti S., Vernadakis A., Freed C., Prasad K. Characterization and transplantation of two neuronal cell lines with dopaminergic properties. Neurochem. Res. 1996;21:619–627. - PubMed
-
- Braak H., Tredici K.D., Rüb U., de Vos R.A.I., Jansen Steur E.N.H., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. - PubMed
-
- Chen H., Wu D., Ding X., Ying W. SIRT2 is required for lipopolysaccharide-induced activation of BV2 microglia. Neuroreport. 2015;26:88–93. - PubMed
-
- Chen P.S., Peng G.S., Li G., Yang S., Wu X., Wang C.C., Wilson B., Lu R.B., Gean P.W., Chuang D.M., Hong J.S. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychiatry. 2006;11:1116–1125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

