Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation
- PMID: 29273652
- PMCID: PMC5778339
- DOI: 10.1136/bmjopen-2017-017157
Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation
Abstract
Objectives: To provide an accurate, web-based tool for stratifying patients with atrial fibrillation to facilitate decisions on the potential benefits/risks of anticoagulation, based on mortality, stroke and bleeding risks.
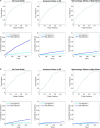
Design: The new tool was developed, using stepwise regression, for all and then applied to lower risk patients. C-statistics were compared with CHA2DS2-VASc using 30-fold cross-validation to control for overfitting. External validation was undertaken in an independent dataset, Outcome Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).
Participants: Data from 39 898 patients enrolled in the prospective GARFIELD-AF registry provided the basis for deriving and validating an integrated risk tool to predict stroke risk, mortality and bleeding risk.
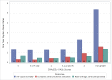
Results: The discriminatory value of the GARFIELD-AF risk model was superior to CHA2DS2-VASc for patients with or without anticoagulation. C-statistics (95% CI) for all-cause mortality, ischaemic stroke/systemic embolism and haemorrhagic stroke/major bleeding (treated patients) were: 0.77 (0.76 to 0.78), 0.69 (0.67 to 0.71) and 0.66 (0.62 to 0.69), respectively, for the GARFIELD-AF risk models, and 0.66 (0.64-0.67), 0.64 (0.61-0.66) and 0.64 (0.61-0.68), respectively, for CHA2DS2-VASc (or HAS-BLED for bleeding). In very low to low risk patients (CHA2DS2-VASc 0 or 1 (men) and 1 or 2 (women)), the CHA2DS2-VASc and HAS-BLED (for bleeding) scores offered weak discriminatory value for mortality, stroke/systemic embolism and major bleeding. C-statistics for the GARFIELD-AF risk tool were 0.69 (0.64 to 0.75), 0.65 (0.56 to 0.73) and 0.60 (0.47 to 0.73) for each end point, respectively, versus 0.50 (0.45 to 0.55), 0.59 (0.50 to 0.67) and 0.55 (0.53 to 0.56) for CHA2DS2-VASc (or HAS-BLED for bleeding). Upon validation in the ORBIT-AF population, C-statistics showed that the GARFIELD-AF risk tool was effective for predicting 1-year all-cause mortality using the full and simplified model for all-cause mortality: C-statistics 0.75 (0.73 to 0.77) and 0.75 (0.73 to 0.77), respectively, and for predicting for any stroke or systemic embolism over 1 year, C-statistics 0.68 (0.62 to 0.74).
Conclusions: Performance of the GARFIELD-AF risk tool was superior to CHA2DS2-VASc in predicting stroke and mortality and superior to HAS-BLED for bleeding, overall and in lower risk patients. The GARFIELD-AF tool has the potential for incorporation in routine electronic systems, and for the first time, permits simultaneous evaluation of ischaemic stroke, mortality and bleeding risks.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier for GARFIELD-AF (NCT01090362) and for ORBIT-AF (NCT01165710).
Keywords: CHA2DS2-VASc; atrial fibrillation; risk stratification.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: KAAF reports grants and personal fees from Bayer, and Johnson and Johnson, personal fees from Lilly, grants and personal fees from AstraZeneca and personal fees from Sanofi/Regeneron outside the submitted work. JEL and KSP have provided statistical support and thought leadership for the Thrombosis Research Institute, during the conduct of the study, and KSP has received personal fees from Bayer outside the submitted work. J-PB reports personal fees from Aspen outside the submitted work. AJC is an advisor to Bayer, Boehringer Ingelheim, Pfizer/BMS and Daiichi Sankyo. DAF reports personal fees from Bayer outside the submitted work. SZG reports grants from BiO2 Medical, Boehringer-Ingelheim, Bristol Meyers Squibb, BTG EKOS, Daiichi Sankyo, National Heart Lung and Blood Institute of the National Institutes of Health, Janssen and Thrombosis Research Group and personal fees from Bayer, Boehringer-Ingelheim, Bristol Meyers Squibb, Daiichi Sankyo, Janssen and Portola outside the submitted work. SG reports personal fees from Bayer, grants from Sanofi, grants from Pfizer, personal fees from Daiichi-Sankyo, personal fees from AstraZeneca during the conduct of the study and grants from Bayer outside the submitted work. SH reports personal fees from Aspen, Bayer AG, BMS, Daiichi-Sankyo, Pfizer and Sanofi outside the submitted work. WH reports personal fees from Bayer during the conduct of the study. GK reports grants from Bayer during the conduct of the study. LGM reports grants and personal fees from Bayer AG during the conduct of the study, and grants from Boehringer Ingelheim, grants and personal fees from Pfizer and personal fees from Daiichi Sankyo outside the submitted work. FM reports grants and other from Bayer AG during the conduct of the study, and other from Bayer AG outside the submitted work. FM is an employee of Bayer AG. JP has nothing to disclose. AGGT reports consultant fees from Bayer AG during the conduct of the study. FWAV reports personal fees from Boehringer-Ingelheim, Bayer AG, BMS/Pfizer and Daiichi-Sankyo during the conduct of the study and personal fees from AstraZeneca outside the submitted work. AKK reports grants and personal fees from Bayer AG, Boehringer-Ingelheim Pharma, Daiichi Sankyo Europe, Sanofi SA and Janssen Pharma outside the submitted work.
Figures

References
-
- Camm AJ, Lip GY, De Caterina R, et al. . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. 10.1093/eurheartj/ehs253 - DOI - PubMed
-
- January CT, Wann LS, Alpert JS, et al. . AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–e267. 10.1161/CIR.0000000000000041 - DOI - PMC - PubMed
-
- Gage BF, Waterman AD, Shannon W, et al. . Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
