Design and rationale of the Cardiovascular Health and Text Messaging (CHAT) Study and the CHAT-Diabetes Mellitus (CHAT-DM) Study: two randomised controlled trials of text messaging to improve secondary prevention for coronary heart disease and diabetes
- PMID: 29273661
- PMCID: PMC5778311
- DOI: 10.1136/bmjopen-2017-018302
Design and rationale of the Cardiovascular Health and Text Messaging (CHAT) Study and the CHAT-Diabetes Mellitus (CHAT-DM) Study: two randomised controlled trials of text messaging to improve secondary prevention for coronary heart disease and diabetes
Erratum in
-
Correction: Design and rationale of the Cardiovascular Health and Text Messaging (CHAT) Study and the CHAT-Diabetes Mellitus (CHAT-DM) Study: two randomised controlled trials of text messaging to improve secondary prevention for coronary heart disease and diabetes.BMJ Open. 2018 Jan 21;8(1):e018302corr1. doi: 10.1136/bmjopen-2017-018302corr1. BMJ Open. 2018. PMID: 29358454 Free PMC article. No abstract available.
Abstract
Introduction: Mobile health interventions have the potential to promote risk factor management and lifestyle modification, and are a particularly attractive approach for scaling across healthcare systems with limited resources. We are conducting two randomised trials to evaluate the efficacy of text message-based health messages in improving secondary coronary heart disease (CHD) prevention among patients with or without diabetes.
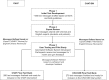
Methods and analysis: The Cardiovascular Health And Text Messaging (CHAT) Study and the CHAT-Diabetes Mellitus (CHAT-DM) Study are multicentre, single-blind, randomised controlled trials of text messaging versus standard treatment with 6 months of follow-up conducted in 37 hospitals throughout 17 provinces in China. The intervention group receives six text messages per week which target blood pressure control, medication adherence, physical activity, smoking cessation (when appropriate), glucose monitoring and lifestyle recommendations including diet (in CHAT-DM). The text messages were developed based on behavioural change techniques, using models such as the information-motivation-behavioural skills model, goal setting and provision of social support. A total sample size of 800 patients would be adequate for CHAT Study and sample size of 500 patients would be adequate for the CHAT-DM Study. In CHAT, the primary outcome is the change in systolic blood pressure (SBP) at 6 months. Secondary outcomes include a change in proportion of patients achieving a SBP <140 mm Hg, low-density lipoprotein cholesterol (LDL-C), physical activity, medication adherence, body mass index (BMI) and smoking cessation. In CHAT-DM, the primary outcome is the change in glycaemic haemoglobin (HbA1C) at 6 months. Secondary outcomes include a change in the proportion of patients achieving HbA1C<7%, fasting blood glucose, SBP, LDL-C, BMI, physical activity and medication adherence.
Ethics and dissemination: The central ethics committee at the China National Center for Cardiovascular Disease and the Yale University Institutional Review Board approved the CHAT and CHAT-DM studies. Results will be disseminated via usual scientific forums including peer-reviewed publications.
Trial registration number: CHAT (NCT02888769) and CHAT-DM (NCT02883842); Pre-results.
Keywords: behavioral intervention; coronary heart disease; diabetes; secondary prevention; text messaging.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: HMK is a recipient of research agreements from Medtronic and Johnson & Johnson (Janssen) through Yale University to develop methods of clinical trial data sharing. He is also the recipient of a grant from the Food and Drug Administration and Medtronic to develop methods for postmarket surveillance of medical devices; and is the founder of Hugo, a personal health information platform. All other authors have no conflicts of interests to declare.
Figures

References
-
- Gaede PH, Jepsen PV, Larsen JN, et al. [The Steno-2 study. Intensive multifactorial intervention reduces the occurrence of cardiovascular disease in patients with type 2 diabetes]. Ugeskr Laeger 2003;165:2658–61. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
