CD1a presentation of endogenous antigens by group 2 innate lymphoid cells
- PMID: 29273672
- PMCID: PMC5826589
- DOI: 10.1126/sciimmunol.aan5918
CD1a presentation of endogenous antigens by group 2 innate lymphoid cells
Abstract
Group 2 innate lymphoid cells (ILC2) are effectors of barrier immunity, with roles in infection, wound healing, and allergy. A proportion of ILC2 express MHCII (major histocompatibility complex II) and are capable of presenting peptide antigens to T cells and amplifying the subsequent adaptive immune response. Recent studies have highlighted the importance of CD1a-reactive T cells in allergy and infection, activated by the presentation of endogenous neolipid antigens and bacterial components. Using a human skin challenge model, we unexpectedly show that human skin-derived ILC2 can express CD1a and are capable of presenting endogenous antigens to T cells. CD1a expression is up-regulated by TSLP (thymic stromal lymphopoietin) at levels observed in the skin of patients with atopic dermatitis, and the response is dependent on PLA2G4A. Furthermore, this pathway is used to sense Staphylococcus aureus by promoting Toll-like receptor-dependent CD1a-reactive T cell responses to endogenous ligands. These findings define a previously unrecognized role for ILC2 in lipid surveillance and identify shared pathways of CD1a- and PLA2G4A-dependent ILC2 inflammation amenable to therapeutic intervention.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
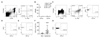

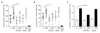


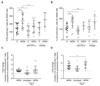


Comment in
-
Innate lymphoid cells: Lipid surveillance by skin ILCs.Nat Rev Immunol. 2018 Feb;18(2):78-79. doi: 10.1038/nri.2018.1. Epub 2018 Jan 15. Nat Rev Immunol. 2018. PMID: 29332938 No abstract available.
References
-
- Shim DH, Park YA, Kim MJ, Hong JY, Baek JY, Kim KW, Byun YH, Seong BL, Ryu S, Song MK, Hong KJ, et al. Pandemic influenza virus, pH1N1, induces asthmatic symptoms via activation of innate lymphoid cells. Pediatr Allergy Immunol. 2015;26:780–788. - PubMed
-
- Bouchery T, Kyle R, Camberis M, Shepherd A, Filbey K, Smith A, Harvie M, Painter G, Johnston K, Ferguson P, Jain R, et al. ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat Commun. 2015;6:6970. - PubMed
-
- Mielke LA, Groom JR, Rankin LC, Seillet C, Masson F, Putoczki T, Belz GT. TCF-1 controls ILC2 and NKp46+RORgammat+ innate lymphocyte differentiation and protection in intestinal inflammation. J Immunol. 2013;191:4383–4391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

