Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture
- PMID: 29273780
- PMCID: PMC5741621
- DOI: 10.1038/s41467-017-02340-3
Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture
Abstract
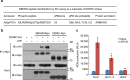
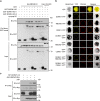
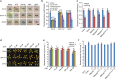
In addition to acting as a cellular energy source, ATP can also act as a damage-associated molecular pattern in both animals and plants. Stomata are leaf pores that control gas exchange and, therefore, impact critical functions such as photosynthesis, drought tolerance, and also are the preferred entry point for pathogens. Here we show the addition of ATP leads to the rapid closure of leaf stomata and enhanced resistance to the bacterial pathogen Psuedomonas syringae. This response is mediated by ATP recognition by the receptor DORN1, followed by direct phosphorylation of the NADPH oxidase RBOHD, resulting in elevated production of reactive oxygen species and stomatal closure. Mutation of DORN1 phosphorylation sites on RBOHD eliminates the ability of ATP to induce stomatal closure. The data implicate purinergic signaling via DORN1 in the control of stomatal aperture with important implications for the control of plant photosynthesis, water homeostasis, pathogen resistance, and ultimately yield.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

