Review of optical coherence tomography in oncology
- PMID: 29274145
- PMCID: PMC5741100
- DOI: 10.1117/1.JBO.22.12.121711
Review of optical coherence tomography in oncology
Abstract
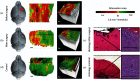
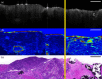
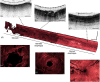
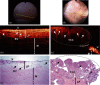
The application of optical coherence tomography (OCT) in the field of oncology has been prospering over the past decade. OCT imaging has been used to image a broad spectrum of malignancies, including those arising in the breast, brain, bladder, the gastrointestinal, respiratory, and reproductive tracts, the skin, and oral cavity, among others. OCT imaging has initially been applied for guiding biopsies, for intraoperatively evaluating tumor margins and lymph nodes, and for the early detection of small lesions that would often not be visible on gross examination, tasks that align well with the clinical emphasis on early detection and intervention. Recently, OCT imaging has been explored for imaging tumor cells and their dynamics, and for the monitoring of tumor responses to treatments. This paper reviews the evolution of OCT technologies for the clinical application of OCT in surgical and noninvasive interventional oncology procedures and concludes with a discussion of the future directions for OCT technologies, with particular emphasis on their applications in oncology.
Keywords: cancer; intraoperative; oncology; optical coherence tomography; surgery; tumor.
(2017) COPYRIGHT Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures



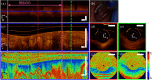

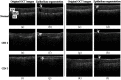
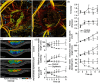
References
-
- Czernin J., Allen-Auerbach M., Schelbert H. R., “Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006,” J. Nucl. Med. 48(1 suppl), 78S–88S (2007). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

