Recent progress in [11 C]carbon dioxide ([11 C]CO2 ) and [11 C]carbon monoxide ([11 C]CO) chemistry
- PMID: 29274276
- PMCID: PMC6485328
- DOI: 10.1002/jlcr.3596
Recent progress in [11 C]carbon dioxide ([11 C]CO2 ) and [11 C]carbon monoxide ([11 C]CO) chemistry
Abstract
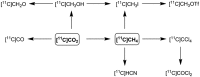
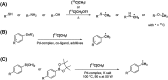
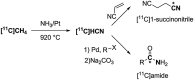
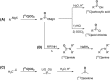
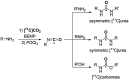
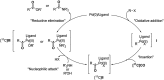
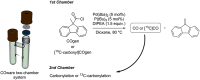
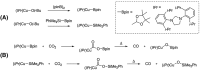
[11 C]Carbon dioxide ([11 C]CO2 ) and [11 C]carbon monoxide ([11 C]CO) are 2 attractive precursors for labelling the carbonyl position (C═O) in a vast range of functionalised molecules (eg, ureas, amides, and carboxylic acids). The development of radiosynthetic methods to produce functionalised 11 C-labelled compounds is required to enhance the radiotracers available for positron emission tomography, molecular, and medical imaging applications. Following a brief summary of secondary 11 C-precursor production and uses, the review focuses on recent progress with direct 11 C-carboxylation routes with [11 C]CO2 and 11 C-carbonylation with [11 C]CO. Novel approaches to generate [11 C]CO using CO-releasing molecules (CO-RMs), such as silacarboxylic acids and disilanes, applied to radiochemistry are described and compared with standard [11 C]CO production methods. These innovative [11 C]CO synthesis strategies represent efficient and reliable [11 C]CO production processes, enabling the widespread use of [11 C]CO chemistry within the wider radiochemistry community.
Keywords: 11C-carbonylation; 11C-carboxylation; 11C-labelling; CO-releasing molecules; PET; [11C]CO; [11C]CO2; carbon-11.
Copyright © 2017 John Wiley & Sons, Ltd.
Conflict of interest statement
None declared.
Figures



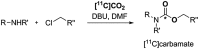

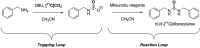
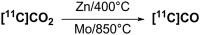

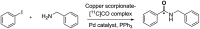






References
-
- McQuade P, Rowland DJ, Lewis JS, Welch MJ. Positron‐emitting isotopes produced on biomedical cyclotrons. Curr Med Chem. 2005;12(7):807‐818. - PubMed
-
- Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem Int Ed. 2008;47(47):8998‐9033. - PubMed
-
- Oleksiy I, Vanessa G‐V, Jordi L, Jacek K. On 11C chemistry reviews—surveying and filling the gaps. Curr Org Chem. 2013;17(19):2067‐2096.
-
- Antoni G, Långström B. Progress in 11C radiochemistry In: Positron Emission Tomography: Basic Sciences. London: Springer London; 2005:223‐236.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

