Calcium phosphate particles stimulate interleukin-1β release from human vascular smooth muscle cells: A role for spleen tyrosine kinase and exosome release
- PMID: 29274344
- PMCID: PMC5823844
- DOI: 10.1016/j.yjmcc.2017.12.007
Calcium phosphate particles stimulate interleukin-1β release from human vascular smooth muscle cells: A role for spleen tyrosine kinase and exosome release
Abstract
Aims: Calcium phosphate (CaP) particle deposits are found in several inflammatory diseases including atherosclerosis and osteoarthritis. CaP, and other forms of crystals and particles, can promote inflammasome formation in macrophages leading to caspase-1 activation and secretion of mature interleukin-1β (IL-1β). Given the close association of small CaP particles with vascular smooth muscle cells (VSMCs) in atherosclerotic fibrous caps, we aimed to determine if CaP particles affected pro-inflammatory signalling in human VSMCs.
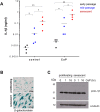
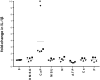
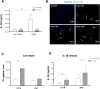
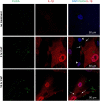
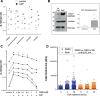
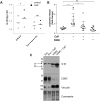
Methods and results: Using ELISA to measure IL-1β release from VSMCs, we demonstrated that CaP particles stimulated IL-1β release from proliferating and senescent human VSMCs, but with substantially greater IL-1β release from senescent cells; this required caspase-1 activity but not LPS-priming of cells. Potential inflammasome agonists including ATP, nigericin and monosodium urate crystals did not stimulate IL-1β release from VSMCs. Western blot analysis demonstrated that CaP particles induced rapid activation of spleen tyrosine kinase (SYK) (increased phospho-Y525/526). The SYK inhibitor R406 reduced IL-1β release and caspase-1 activation in CaP particle-treated VSMCs, indicating that SYK activation occurs upstream of and is required for caspase-1 activation. In addition, IL-1β and caspase-1 colocalised in intracellular endosome-like vesicles and we detected IL-1β in exosomes isolated from VSMC media. Furthermore, CaP particle treatment stimulated exosome secretion by VSMCs in a SYK-dependent manner, while the exosome-release inhibitor spiroepoxide reduced IL-1β release.
Conclusions: CaP particles stimulate SYK and caspase-1 activation in VSMCs, leading to the release of IL-1β, at least in part via exosomes. These novel findings in human VSMCs highlight the pro-inflammatory and pro-calcific potential of microcalcification.
Keywords: Calcium phosphate particles; Caspase-1; Cytokines; Exosomes; SYK; Vascular smooth muscle.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
NLRP3 Inflammasome Activation Controls Vascular Smooth Muscle Cells Phenotypic Switch in Atherosclerosis.Int J Mol Sci. 2021 Dec 29;23(1):340. doi: 10.3390/ijms23010340. Int J Mol Sci. 2021. PMID: 35008765 Free PMC article.
-
Cytolethal distending toxin-induced release of interleukin-1β by human macrophages is dependent upon activation of glycogen synthase kinase 3β, spleen tyrosine kinase (Syk) and the noncanonical inflammasome.Cell Microbiol. 2020 Jul;22(7):e13194. doi: 10.1111/cmi.13194. Epub 2020 Mar 4. Cell Microbiol. 2020. PMID: 32068949 Free PMC article.
-
Inhibition of vascular neointima hyperplasia by FGF21 associated with FGFR1/Syk/NLRP3 inflammasome pathway in diabetic mice.Atherosclerosis. 2019 Oct;289:132-142. doi: 10.1016/j.atherosclerosis.2019.08.017. Epub 2019 Aug 29. Atherosclerosis. 2019. PMID: 31513948
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation.J Physiol. 2016 Jun 1;594(11):2905-14. doi: 10.1113/JP271340. Epub 2016 Mar 9. J Physiol. 2016. PMID: 26864864 Free PMC article. Review.
Cited by
-
Delivery of extracellular vesicles loaded with immune checkpoint inhibitors for immunotherapeutic management of glioma.Mater Today Bio. 2024 Sep 14;28:101244. doi: 10.1016/j.mtbio.2024.101244. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39318378 Free PMC article.
-
Development of Neovasculature in Axially Vascularized Calcium Phosphate Cement Scaffolds.J Funct Biomater. 2023 Feb 14;14(2):105. doi: 10.3390/jfb14020105. J Funct Biomater. 2023. PMID: 36826904 Free PMC article.
-
Role of Uremic Toxins in Early Vascular Ageing and Calcification.Toxins (Basel). 2021 Jan 3;13(1):26. doi: 10.3390/toxins13010026. Toxins (Basel). 2021. PMID: 33401534 Free PMC article. Review.
-
ECM Modifications Driven by Age and Metabolic Stress Directly Promote Vascular Smooth Muscle Cell Osteogenic Processes.Arterioscler Thromb Vasc Biol. 2025 Mar;45(3):424-442. doi: 10.1161/ATVBAHA.124.321467. Epub 2025 Jan 16. Arterioscler Thromb Vasc Biol. 2025. PMID: 39817328 Free PMC article.
-
Uremic Toxins and Vascular Calcification-Missing the Forest for All the Trees.Toxins (Basel). 2020 Sep 29;12(10):624. doi: 10.3390/toxins12100624. Toxins (Basel). 2020. PMID: 33003628 Free PMC article. Review.
References
-
- Virmani R., Burke A.P., Kolodgie F.D., Farb A. Pathology of the thin-cap fibroatheroma: a type of vulnerable plaque. J. Interv. Cardiol. 2003;16(3):267–272. - PubMed
-
- Ehara S., Kobayashi Y., Yoshiyama M., Shimada K., Shimada Y., Fukuda D. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110(22):3424–3429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

