A Heparin Binding Motif Rich in Arginine and Lysine is the Functional Domain of YKL-40
- PMID: 29274508
- PMCID: PMC5773473
- DOI: 10.1016/j.neo.2017.11.011
A Heparin Binding Motif Rich in Arginine and Lysine is the Functional Domain of YKL-40
Abstract
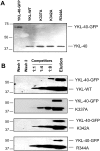
The heparin-binding glycoprotein YKL-40 (CHI3L1) is intimately associated with microvascularization in multiple human diseases including cancer and inflammation. However, the heparin-binding domain(s) pertinent to the angiogenic activity have yet been identified. YKL-40 harbors a consensus heparin-binding motif that consists of positively charged arginine (R) and lysine (K) (RRDK; residues 144-147); but they don't bind to heparin. Intriguingly, we identified a separate KR-rich domain (residues 334-345) that does display strong heparin binding affinity. A short synthetic peptide spanning this KR-rich domain successfully competed with YKL-40 and blocked its ability to bind heparin. Three individual point mutations, where alanine (A) substituted for K or R (K337A, K342A, R344A), led to remarkable decreases in heparin-binding ability and angiogenic activity. In addition, a neutralizing anti-YKL-40 antibody that targets these residues and prevents heparin binding impeded angiogenesis in vitro. MDA-MB-231 breast cancer cells engineered to express ectopic K337A, K342A or R344A mutants displayed reduced tumor development and compromised tumor vessel formation in mice relative to control cells expressing wild-type YKL-40. These data reveal that the KR-rich heparin-binding motif is the functional heparin-binding domain of YKL-40. Our findings shed light on novel molecular mechanisms underlying endothelial cell angiogenesis promoted by YKL-40 in a variety of diseases.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
YKL-40, a secreted glycoprotein, promotes tumor angiogenesis.Oncogene. 2009 Dec 17;28(50):4456-68. doi: 10.1038/onc.2009.292. Epub 2009 Sep 21. Oncogene. 2009. PMID: 19767768 Free PMC article.
-
A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers.Mol Cancer Ther. 2011 May;10(5):742-51. doi: 10.1158/1535-7163.MCT-10-0868. Epub 2011 Feb 25. Mol Cancer Ther. 2011. PMID: 21357475 Free PMC article.
-
YKL-40/CHI3L1 facilitates migration and invasion in HER2 overexpressing breast epithelial progenitor cells and generates a niche for capillary-like network formation.In Vitro Cell Dev Biol Anim. 2019 Dec;55(10):838-853. doi: 10.1007/s11626-019-00403-x. Epub 2019 Sep 3. In Vitro Cell Dev Biol Anim. 2019. PMID: 31482369 Free PMC article.
-
Angiogenic potential of YKL-40 in the dynamics of tumor niche.Biomed Pharmacother. 2018 Apr;100:478-485. doi: 10.1016/j.biopha.2018.02.050. Epub 2018 Feb 23. Biomed Pharmacother. 2018. PMID: 29477911 Review.
-
The role of YKL-40 in the pathogenesis of autoimmune diseases: a comprehensive review.Int J Biol Sci. 2022 May 21;18(9):3731-3746. doi: 10.7150/ijbs.67587. eCollection 2022. Int J Biol Sci. 2022. PMID: 35813465 Free PMC article. Review.
Cited by
-
Uncovering novel mechanisms of chitinase-3-like protein 1 in driving inflammation-associated cancers.Cancer Cell Int. 2024 Jul 27;24(1):268. doi: 10.1186/s12935-024-03425-y. Cancer Cell Int. 2024. PMID: 39068486 Free PMC article. Review.
-
Inflammatory Biomarker Score Identifies Patients with Six-Fold Increased Risk of One-Year Mortality after Pancreatic Cancer.Cancers (Basel). 2021 Sep 13;13(18):4599. doi: 10.3390/cancers13184599. Cancers (Basel). 2021. PMID: 34572824 Free PMC article.
-
Serum IL6 as a Prognostic Biomarker and IL6R as a Therapeutic Target in Biliary Tract Cancers.Clin Cancer Res. 2020 Nov 1;26(21):5655-5667. doi: 10.1158/1078-0432.CCR-19-2700. Epub 2020 Sep 15. Clin Cancer Res. 2020. PMID: 32933994 Free PMC article.
-
Human chitinases and chitinase-like proteins as emerging drug targets - a medicinal chemistry perspective.RSC Med Chem. 2025 Apr 24;16(6):2388-2402. doi: 10.1039/d4md01050g. eCollection 2025 Jun 18. RSC Med Chem. 2025. PMID: 40313579 Free PMC article. Review.
-
Prodrug-conjugated tumor-seeking commensals for targeted cancer therapy.Nat Commun. 2024 May 21;15(1):4343. doi: 10.1038/s41467-024-48661-y. Nat Commun. 2024. PMID: 38773197 Free PMC article.
References
-
- Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–509. - PubMed
-
- Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra BW. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J Biol Chem. 2003;278:37753–37760. - PubMed
-
- Hu B, Trinh K, Figueira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J Biol Chem. 1996;271:19415–19420. - PubMed
-
- Shackelton LM, Mann DM, Millis AJ. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem. 1995;270:13076–13083. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

