Large-scale production of a thermostable Rhodothermus marinus cellulase by heterologous secretion from Streptomyces lividans
- PMID: 29274637
- PMCID: PMC5741968
- DOI: 10.1186/s12934-017-0847-x
Large-scale production of a thermostable Rhodothermus marinus cellulase by heterologous secretion from Streptomyces lividans
Abstract
Background: The gene encoding a thermostable cellulase of family 12 was previously isolated from a Rhodothermus marinus through functional screening. CelA is a protein of 260 aminoacyl residues with a 28-residue amino-terminal signal peptide. Mature CelA was poorly synthesized in some Escherichia coli strains and not at all in others. Here we present an alternative approach for its heterologous production as a secreted polypeptide in Streptomyces.
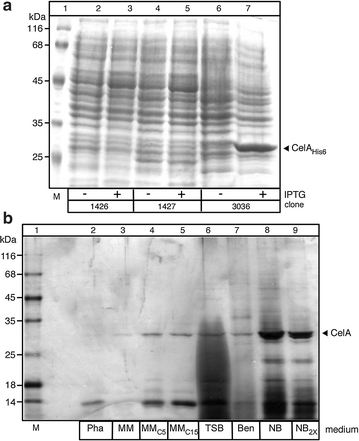
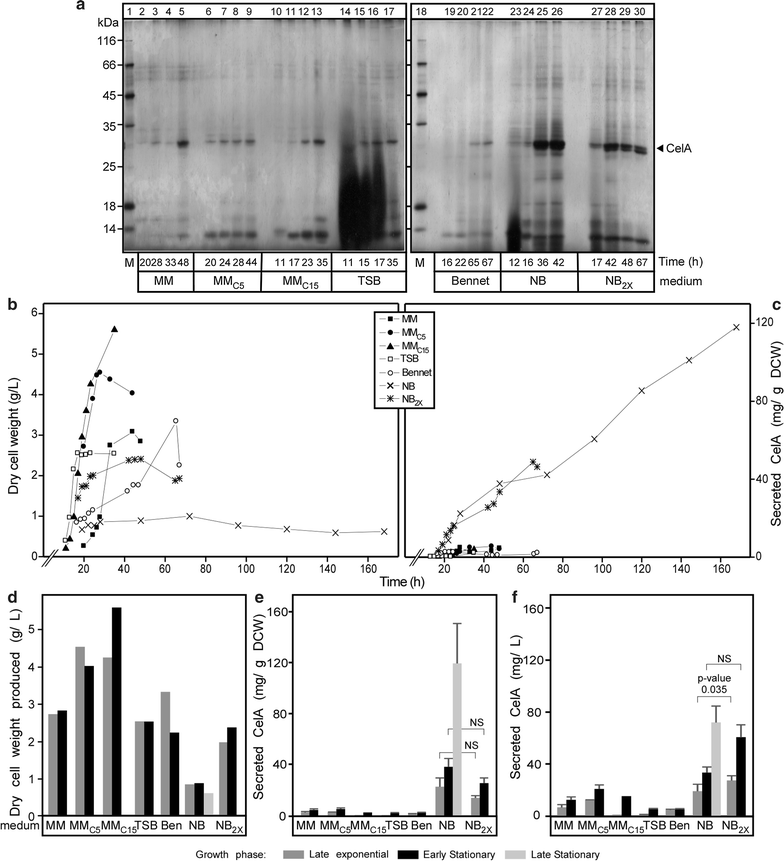
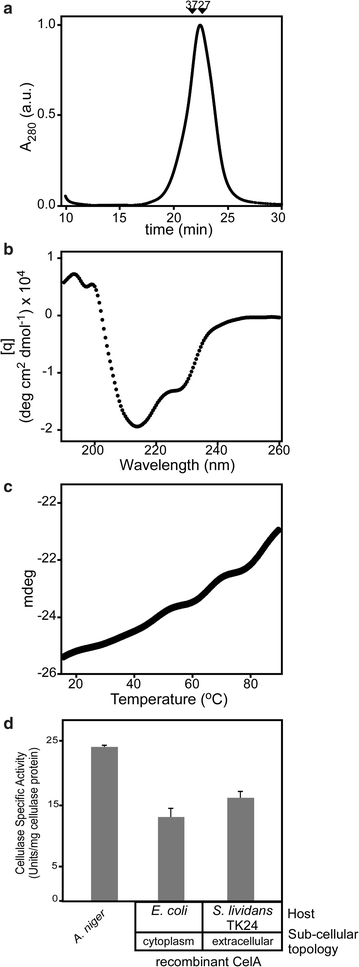
Results: CelA was successfully over-expressed as a secreted polypeptide in Streptomyces lividans TK24. To this end, CelA was fused C-terminally to the secretory signal peptide of the subtilisin inhibitor protein (Sianidis et al. in J Biotechnol. 121: 498-507, 2006) from Streptomyces venezuelae and a new cloning strategy developed. Optimal growth media and conditions that stall biomass production promote excessive CelA secretion. Under optimal growth conditions in nutrient broth medium, significant amounts of mature CelA (50-90 mg/L or 100-120 mg/g of dry cell weight) are secreted in the spent growth media after 7 days. A protocol to rapidly purify CelA to homogeneity from culture supernatants was developed and specific anti-sera raised against it. Biophysical, biochemical and immmuno-detection analyses indicate that the enzyme is intact, stable and fully functional. CelA is the most thermostable heterologous polypeptide shown to be secreted from S. lividans.
Conclusion: This study further validates and extends the use of the S. lividans platform for production of heterologous enzymes of industrial importance and extends it to active thermostable enzymes. This study contributes to developing a platform for poly-omics analysis of protein secretion in S. lividans.
Keywords: Cellulase; Protein secretion biotechnology; Protein translocase; Secretion; Signal peptide; Streptomyces lividans.
Figures


Similar articles
-
Transcriptomic and fluxomic changes in Streptomyces lividans producing heterologous protein.Microb Cell Fact. 2018 Dec 21;17(1):198. doi: 10.1186/s12934-018-1040-6. Microb Cell Fact. 2018. PMID: 30577858 Free PMC article.
-
Functional large-scale production of a novel Jonesia sp. xyloglucanase by heterologous secretion from Streptomyces lividans.J Biotechnol. 2006 Feb 24;121(4):498-507. doi: 10.1016/j.jbiotec.2005.08.002. Epub 2005 Sep 15. J Biotechnol. 2006. PMID: 16168511
-
Over-production of various secretory-form proteins in Streptomyces lividans.Protein Expr Purif. 2010 Oct;73(2):198-202. doi: 10.1016/j.pep.2010.05.011. Epub 2010 May 31. Protein Expr Purif. 2010. PMID: 20546899
-
Streptomyces protein secretion and its application in biotechnology.FEMS Microbiol Lett. 2018 Nov 1;365(22). doi: 10.1093/femsle/fny250. FEMS Microbiol Lett. 2018. PMID: 30299471 Review.
-
Protein secretion biotechnology in Gram-positive bacteria with special emphasis on Streptomyces lividans.Biochim Biophys Acta. 2014 Aug;1843(8):1750-61. doi: 10.1016/j.bbamcr.2013.12.023. Epub 2014 Jan 9. Biochim Biophys Acta. 2014. PMID: 24412306 Review.
Cited by
-
Transcriptomic and fluxomic changes in Streptomyces lividans producing heterologous protein.Microb Cell Fact. 2018 Dec 21;17(1):198. doi: 10.1186/s12934-018-1040-6. Microb Cell Fact. 2018. PMID: 30577858 Free PMC article.
-
Comprehensive subcellular topologies of polypeptides in Streptomyces.Microb Cell Fact. 2018 Mar 15;17(1):43. doi: 10.1186/s12934-018-0892-0. Microb Cell Fact. 2018. PMID: 29544487 Free PMC article.
-
A novel autolysis system for extracellular production and direct immobilization of a phospholipase D fused with cellulose binding domain.BMC Biotechnol. 2019 May 22;19(1):29. doi: 10.1186/s12896-019-0519-5. BMC Biotechnol. 2019. PMID: 31118018 Free PMC article.
-
Streptomycetes: Attractive Hosts for Recombinant Protein Production.Front Microbiol. 2020 Aug 20;11:1958. doi: 10.3389/fmicb.2020.01958. eCollection 2020. Front Microbiol. 2020. PMID: 32973711 Free PMC article. Review.
-
Secretome Dynamics in a Gram-Positive Bacterial Model.Mol Cell Proteomics. 2019 Mar;18(3):423-436. doi: 10.1074/mcp.RA118.000899. Epub 2018 Nov 29. Mol Cell Proteomics. 2019. PMID: 30498012 Free PMC article.
References
-
- Sianidis G, Pozidis C, Becker F, Vrancken K, Sjoeholm C, Karamanou S, Takamiya-Wik M, van Mellaert L, Schaefer T, Anne J, Economou A. Functional large-scale production of a novel Jonesia sp. xyloglucanase by heterologous secretion from Streptomyces lividans. J Biotechnol. 2006;121:498–507. doi: 10.1016/j.jbiotec.2005.08.002. - DOI - PubMed
-
- Lara M, Servin-Gonzalez L, Singh M, Moreno C, Cohen I, Nimtz M, Espitia C. Expression, secretion, and glycosylation of the 45- and 47-kDa glycoprotein of Mycobacterium tuberculosis in Streptomyces lividans. Appl Environ Microbiol. 2004;70:679–685. doi: 10.1128/AEM.70.2.679-685.2004. - DOI - PMC - PubMed
-
- Pozidis C, Lammertyn E, Politou AS, Anné J, Tsiftsoglou AS, Sianidis G, Economou A. Protein secretion biotechnology using Streptomyces lividans: large-scale production of functional trimeric tumor necrosis factor alpha. Biotechnol Bioeng. 2001;72:611–619. doi: 10.1002/1097-0290(20010320)72:6<611::AID-BIT1026>3.0.CO;2-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

