Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation
- PMID: 29275332
- PMCID: PMC5890624
- DOI: 10.1136/annrheumdis-2017-212127
Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation
Abstract
Objective: Interleukin (IL)-17A has emerged as pivotal in driving tissue pathology in immune-mediated inflammatory diseases. The role of IL-17F, sharing 50% sequence homology and overlapping biological function, remains less clear. We hypothesised that IL-17F, together with IL-17A, contributes to chronic tissue inflammation, and that dual neutralisation may lead to more profound suppression of inflammation than inhibition of IL-17A alone.
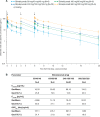
Methods: Preclinical experiments assessed the role of IL-17A and IL-17F in tissue inflammation using disease-relevant human cells. A placebo-controlled proof-of-concept (PoC) clinical trial randomised patients with psoriatic arthritis (PsA) to bimekizumab (n=39) or placebo (n=14). Safety, pharmacokinetics and clinical efficacy of multiple doses (weeks 0, 3, 6 (240 mg/160 mg/160 mg; 80 mg/40 mg/40 mg; 160 mg/80 mg/80 mg and 560 mg/320 mg/320 mg)) of bimekizumab, a humanised monoclonal IgG1 antibody neutralising both IL-17A and IL-17F, were investigated.
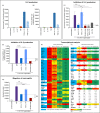
Results: IL-17F induced qualitatively similar inflammatory responses to IL-17A in skin and joint cells. Neutralisation of IL-17A and IL-17F with bimekizumab more effectively suppressed in vitro cytokine responses and neutrophil chemotaxis than inhibition of IL-17A or IL-17F alone. The PoC trial met both prespecified efficacy success criteria and showed rapid, profound responses in both joint and skin (pooled top three doses vs placebo at week 8: American College of Rheumatology 20% response criteria 80.0% vs 16.7% (posterior probability >99%); Psoriasis Area and Severity Index 100% response criteria 86.7% vs 0%), sustained to week 20, without unexpected safety signals.
Conclusions: These data support IL-17F as a key driver of human chronic tissue inflammation and the rationale for dual neutralisation of IL-17A and IL-17F in PsA and related conditions.
Trial registration number: NCT02141763; Results.
Keywords: autoimmune diseases; cytokines; inflammation; psoriatic arthritis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: DB, TB, MG, SG, LI, ADGL, AM, RO, SS, FS, PV, MILW are employees of UCB Pharma. DB, TB, ADGL, PV hold stocks and/or stock options in UCB Pharma. DB is a part-time employee of UCB Pharma and holds a part-time position at the Academic Medical Center/University of Amsterdam. DB received a grant from UCB Pharma to conduct preclinical experiments; DB received grants and/or consultant or investigator fees from the following organizations outside of the submitted work: AbbVie, Pfizer, MSD, Roche, BMS, Novartis, Eli Lilly, Boehringer Ingelheim and Glenmark. MG is a paid contractor for UCB working in a consulting capacity. PM is a scientific advisor to UCB Pharma and received associated fees outside of the submitted work. SP, NY declare no relevant conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

