Potential microRNA-related Targets for Therapeutic Intervention with Ovarian Cancer Metastasis
- PMID: 29275359
- PMCID: PMC5822180
- DOI: 10.21873/cgp.20061
Potential microRNA-related Targets for Therapeutic Intervention with Ovarian Cancer Metastasis
Abstract
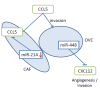
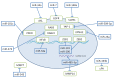
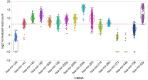
Treatment of disseminated epithelial ovarian cancer (EOC) is an unmet medical need. Therefore, the identification along with preclinical and clinical validation of new targets is an issue of high importance. In this review we focus on microRNAs that mediate metastasis of EOC. We summarize up-regulated metastasis-promoting and down-regulated metastasis-suppressing microRNAs. We focus on preclinical in vitro and in vivo functions as well as their metastasis-related clinical correlations. Finally, we outline modalities for therapeutic intervention and critical issues of microRNA-based therapeutics in the context of metastatic EOC.
Keywords: Antisense oligonucleotides; ascites fluid; epithelial mesenchymal transition; epithelial ovarian cancer; extracellular matrix; locked nucleic acids; mesothelial cells; peritoneal dissemination; review.
Copyright© 2018, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Figures
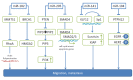
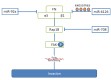
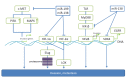
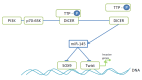
References
-
- American Cancer Society Facts and Figures 2016: Atlanta GA. American Cancer Society. 2016
-
- Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
