The Role of micro RNAs in Breast Cancer Metastasis: Preclinical Validation and Potential Therapeutic Targets
- PMID: 29275360
- PMCID: PMC5822183
- DOI: 10.21873/cgp.20062
The Role of micro RNAs in Breast Cancer Metastasis: Preclinical Validation and Potential Therapeutic Targets
Abstract
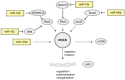
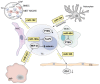
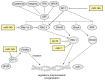
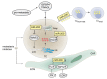
Despite the approval of several molecular therapies in the last years, breast cancer-associated death ranks as the second highest in women. This is due to metastatic disease, which represents a challenge for treatment. A better understanding of the molecular mechanisms of metastasis is, therefore, of paramount importance. In this review we summarize the role of micro RNAs (miRs) involved in metastasis of breast cancer. We present an overview on metastasis-promoting, -suppressing and context-dependent miRs with both activities. We have categorized the corresponding miRs according to their target classes, interaction with stromal cells or exosomes. The pathways affected by individual miRs are outlined in regard to in vitro properties, activity in metastasis-related in vivo models and clinical significance. Current approaches that may be suitable for therapeutic inhibition or restauration of miR activity are outlined. Finally, we discuss the delivery bottlenecks which present as a major challenge in nucleic acid (miR)-based therapies.
Keywords: Colonization of distinct organs; epithelial-mesenchymal transition (EMT); exosomes; mesenchymal-epithelial transition (MET); metastasis-related in vivo models; migration and invasion; review; tumor cell/stromal cell interactions.
Copyright© 2018, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Figures



References
-
- American Cancer Society Breast Cancer Facts and Figures 2015-2016. Atlanta. American Cancer Society Inc. 2015
-
- Santa-Maria CA, Gradishar WJ. Changing treatment paradigms in metastatic breast cancer: lessons learned. JAMA Oncol. 2015;1:528–523. - PubMed
-
- Amedos M, Vicier C, Loi S, Lefebre C, Michels S, Bonnefoi H, Andre F. Precision medicine for metastatic breast cancer-limitations and solutions. Nat Rev Clin Oncol. 2015;12:693–704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
