Nitric Oxide-Dependent Feedback Loop Regulates Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Cooperativity and Endothelial Function in Small Pulmonary Arteries
- PMID: 29275372
- PMCID: PMC5779028
- DOI: 10.1161/JAHA.117.007157
Nitric Oxide-Dependent Feedback Loop Regulates Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Cooperativity and Endothelial Function in Small Pulmonary Arteries
Abstract
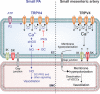
Background: Recent studies demonstrate that spatially restricted, local Ca2+ signals are key regulators of endothelium-dependent vasodilation in systemic circulation. There are drastic functional differences between pulmonary arteries (PAs) and systemic arteries, but the local Ca2+ signals that control endothelium-dependent vasodilation of PAs are not known. Localized, unitary Ca2+ influx events through transient receptor potential vanilloid 4 (TRPV4) channels, termed TRPV4 sparklets, regulate endothelium-dependent vasodilation in resistance-sized mesenteric arteries via activation of Ca2+-dependent K+ channels. The objective of this study was to determine the unique functional roles, signaling targets, and endogenous regulators of TRPV4 sparklets in resistance-sized PAs.
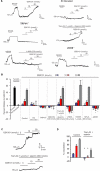
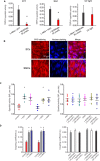
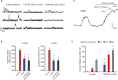
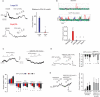
Methods and results: Using confocal imaging, custom image analysis, and pressure myography in fourth-order PAs in conjunction with knockout mouse models, we report a novel Ca2+ signaling mechanism that regulates endothelium-dependent vasodilation in resistance-sized PAs. TRPV4 sparklets exhibit distinct spatial localization in PAs when compared with mesenteric arteries, and preferentially activate endothelial nitric oxide synthase (eNOS). Nitric oxide released by TRPV4-endothelial nitric oxide synthase signaling not only promotes vasodilation, but also initiates a guanylyl cyclase-protein kinase G-dependent negative feedback loop that inhibits cooperative openings of TRPV4 channels, thus limiting sparklet activity. Moreover, we discovered that adenosine triphosphate dilates PAs through a P2 purinergic receptor-dependent activation of TRPV4 sparklets.
Conclusions: Our results reveal a spatially distinct TRPV4-endothelial nitric oxide synthase signaling mechanism and its novel endogenous regulators in resistance-sized PAs.
Keywords: calcium channel; calcium signaling; endothelial nitric oxide synthase; endothelium; microcirculation; pulmonary artery; signaling pathways; transient receptor potential vanilloid 4 channel; vascular endothelial function.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

