Optical coherence elastography in ophthalmology
- PMID: 29275544
- PMCID: PMC5745712
- DOI: 10.1117/1.JBO.22.12.121720
Optical coherence elastography in ophthalmology
Abstract
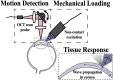
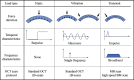
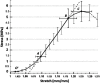
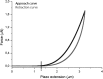
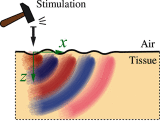
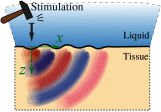
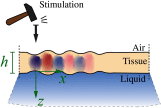
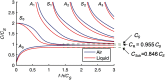
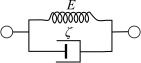
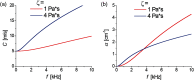
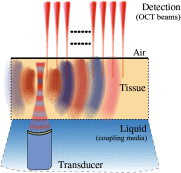
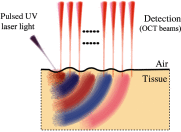
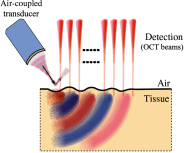
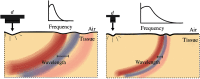
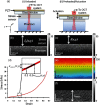
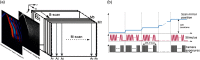
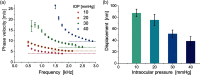
Optical coherence elastography (OCE) can provide clinically valuable information based on local measurements of tissue stiffness. Improved light sources and scanning methods in optical coherence tomography (OCT) have led to rapid growth in systems for high-resolution, quantitative elastography using imaged displacements and strains within soft tissue to infer local mechanical properties. We describe in some detail the physical processes underlying tissue mechanical response based on static and dynamic displacement methods. Namely, the assumptions commonly used to interpret displacement and strain measurements in terms of tissue elasticity for static OCE and propagating wave modes in dynamic OCE are discussed with the ultimate focus on OCT system design for ophthalmic applications. Practical OCT motion-tracking methods used to map tissue elasticity are also presented to fully describe technical developments in OCE, particularly noting those focused on the anterior segment of the eye. Clinical issues and future directions are discussed in the hope that OCE techniques will rapidly move forward to translational studies and clinical applications.
Keywords: air-coupled ultrasound; mechanical wave imaging; ocular biomechanics; optical coherence elastography, acoustic radiation force; optical coherence tomography; phase-sensitive optical coherence tomography; speckle tracking; tissue elasticity.
(2017) COPYRIGHT Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures
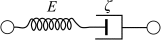






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

