Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses
- PMID: 29275847
- PMCID: PMC5835025
- DOI: 10.1016/j.ymthe.2017.11.017
Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses
Abstract
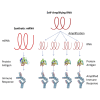
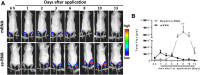
New vaccine platforms are needed to address the time gap between pathogen emergence and vaccine licensure. RNA-based vaccines are an attractive candidate for this role: they are safe, are produced cell free, and can be rapidly generated in response to pathogen emergence. Two RNA vaccine platforms are available: synthetic mRNA molecules encoding only the antigen of interest and self-amplifying RNA (sa-RNA). sa-RNA is virally derived and encodes both the antigen of interest and proteins enabling RNA vaccine replication. Both platforms have been shown to induce an immune response, but it is not clear which approach is optimal. In the current studies, we compared synthetic mRNA and sa-RNA expressing influenza virus hemagglutinin. Both platforms were protective, but equivalent levels of protection were achieved using 1.25 μg sa-RNA compared to 80 μg mRNA (64-fold less material). Having determined that sa-RNA was more effective than mRNA, we tested hemagglutinin from three strains of influenza H1N1, H3N2 (X31), and B (Massachusetts) as sa-RNA vaccines, and all protected against challenge infection. When sa-RNA was combined in a trivalent formulation, it protected against sequential H1N1 and H3N2 challenges. From this we conclude that sa-RNA is a promising platform for vaccines against viral diseases.
Keywords: DNA; H1N1; RNA; alphavirus; influenza; replicon; trivalent; vaccine.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



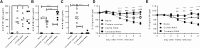

References
-
- Tregoning J.S., Kinnear E. Using plasmids as DNA vaccines for infectious diseases. Microbiol. Spectr. 2014;2:2. - PubMed
-
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
-
- Petsch B., Schnee M., Vogel A.B., Lange E., Hoffmann B., Voss D., Schlake T., Thess A., Kallen K.J., Stitz L., Kramps T. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012;30:1210–1216. - PubMed
-
- Kuhn A.N., Diken M., Kreiter S., Selmi A., Kowalska J., Jemielity J., Darzynkiewicz E., Huber C., Türeci O., Sahin U. Phosphorothioate cap analogs increase stability and translational efficiency of RNA vaccines in immature dendritic cells and induce superior immune responses in vivo. Gene Ther. 2010;17:961–971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

