Molecular Mechanisms for CFIm-Mediated Regulation of mRNA Alternative Polyadenylation
- PMID: 29276085
- PMCID: PMC5756121
- DOI: 10.1016/j.molcel.2017.11.031
Molecular Mechanisms for CFIm-Mediated Regulation of mRNA Alternative Polyadenylation
Abstract
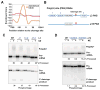
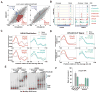
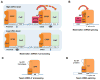
Alternative mRNA processing is a critical mechanism for proteome expansion and gene regulation in higher eukaryotes. The SR family proteins play important roles in splicing regulation. Intriguingly, mammalian genomes encode many poorly characterized SR-like proteins, including subunits of the mRNA 3'-processing factor CFIm, CFIm68 and CFIm59. Here we demonstrate that CFIm functions as an enhancer-dependent activator of mRNA 3' processing. CFIm regulates global alternative polyadenylation (APA) by specifically binding and activating enhancer-containing poly(A) sites (PASs). Importantly, the CFIm activator functions are mediated by the arginine-serine repeat (RS) domains of CFIm68/59, which bind specifically to an RS-like region in the CPSF subunit Fip1, and this interaction is inhibited by CFIm68/59 hyper-phosphorylation. The remarkable functional similarities between CFIm and SR proteins suggest that interactions between RS-like domains in regulatory and core factors may provide a common activation mechanism for mRNA 3' processing, splicing, and potentially other steps in RNA metabolism.
Keywords: RNA-binding proteins; SR proteins; alternative polyadenylation; cleavage; mRNA 3′ processing; polyadenylation; splicing.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Evidence that cleavage factor Im is a heterotetrameric protein complex controlling alternative polyadenylation.Genes Cells. 2010 Sep 1;15(9):1003-13. doi: 10.1111/j.1365-2443.2010.01436.x. Epub 2010 Jul 29. Genes Cells. 2010. PMID: 20695905
-
SRSF3 and SRSF7 modulate 3'UTR length through suppression or activation of proximal polyadenylation sites and regulation of CFIm levels.Genome Biol. 2021 Mar 11;22(1):82. doi: 10.1186/s13059-021-02298-y. Genome Biol. 2021. PMID: 33706811 Free PMC article.
-
Evidence that a threshold of serine/arginine-rich (SR) proteins recruits CFIm to promote rous sarcoma virus mRNA 3' end formation.Virology. 2016 Nov;498:181-191. doi: 10.1016/j.virol.2016.08.021. Epub 2016 Sep 4. Virology. 2016. PMID: 27596537 Free PMC article.
-
Cleavage factor Im (CFIm) as a regulator of alternative polyadenylation.Biochem Soc Trans. 2016 Aug 15;44(4):1051-7. doi: 10.1042/BST20160078. Biochem Soc Trans. 2016. PMID: 27528751 Review.
-
The structure of human cleavage factor I(m) hints at functions beyond UGUA-specific RNA binding: a role in alternative polyadenylation and a potential link to 5' capping and splicing.RNA Biol. 2011 Sep-Oct;8(5):748-53. doi: 10.4161/rna.8.5.16040. Epub 2011 Sep 1. RNA Biol. 2011. PMID: 21881408 Free PMC article. Review.
Cited by
-
CPSF6 links alternative polyadenylation to metabolism adaption in hepatocellular carcinoma progression.J Exp Clin Cancer Res. 2021 Mar 1;40(1):85. doi: 10.1186/s13046-021-01884-z. J Exp Clin Cancer Res. 2021. PMID: 33648552 Free PMC article.
-
Downregulation of CPSF6 leads to global mRNA 3' UTR shortening and enhanced antiviral immune responses.PLoS Pathog. 2024 Feb 28;20(2):e1012061. doi: 10.1371/journal.ppat.1012061. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38416782 Free PMC article.
-
Analysis of SINE Families B2, Dip, and Ves with Special Reference to Polyadenylation Signals and Transcription Terminators.Int J Mol Sci. 2021 Sep 13;22(18):9897. doi: 10.3390/ijms22189897. Int J Mol Sci. 2021. PMID: 34576060 Free PMC article.
-
Fateful Decisions of Where to Cut the Line: Pathology Associated with Aberrant 3' End Processing and Transcription Termination.J Mol Biol. 2025 Jan 1;437(1):168802. doi: 10.1016/j.jmb.2024.168802. Epub 2024 Sep 24. J Mol Biol. 2025. PMID: 39321865 Free PMC article. Review.
-
Co-transcriptional Loading of RNA Export Factors Shapes the Human Transcriptome.Mol Cell. 2019 Jul 25;75(2):310-323.e8. doi: 10.1016/j.molcel.2019.04.034. Epub 2019 May 16. Mol Cell. 2019. PMID: 31104896 Free PMC article.
References
-
- Baron-Benhamou J, Gehring NH, Kulozik AE, Hentze MW. Using the lambdaN peptide to tether proteins to RNAs. Methods Mol Biol. 2004;257:135–154. - PubMed
-
- Brown KM, Gilmartin GM. A mechanism for the regulation of pre-mRNA 3′ processing by human cleavage factor Im. Mol Cell. 2003;12:1467–1476. - PubMed
-
- Cao W, Garcia-Blanco MA. A serine/arginine-rich domain in the human U1 70k protein is necessary and sufficient for ASF/SF2 binding. J Biol Chem. 1998;273:20629–20635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

