Dysbiosis-Associated Change in Host Metabolism Generates Lactate to Support Salmonella Growth
- PMID: 29276172
- PMCID: PMC5764812
- DOI: 10.1016/j.chom.2017.11.006
Dysbiosis-Associated Change in Host Metabolism Generates Lactate to Support Salmonella Growth
Erratum in
-
Dysbiosis-Associated Change in Host Metabolism Generates Lactate to Support Salmonella Growth.Cell Host Microbe. 2018 Apr 11;23(4):570. doi: 10.1016/j.chom.2018.03.013. Cell Host Microbe. 2018. PMID: 29649446 Free PMC article. No abstract available.
Abstract
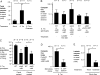
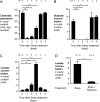
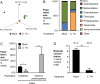
During Salmonella-induced gastroenteritis, mucosal inflammation creates a niche that favors the expansion of the pathogen population over the microbiota. Here, we show that Salmonella Typhimurium infection was accompanied by dysbiosis, decreased butyrate levels, and substantially elevated lactate levels in the gut lumen. Administration of a lactate dehydrogenase inhibitor blunted lactate production in germ-free mice, suggesting that lactate was predominantly of host origin. Depletion of butyrate-producing Clostridia, either through oral antibiotic treatment or as part of the pathogen-induced dysbiosis, triggered a switch in host cells from oxidative metabolism to lactate fermentation, increasing both lactate levels and Salmonella lactate utilization. Administration of tributyrin or a PPARγ agonist diminished host lactate production and abrogated the fitness advantage conferred on Salmonella by lactate utilization. We conclude that alterations of the gut microbiota, specifically a depletion of Clostridia, reprogram host metabolism to perform lactate fermentation, thus supporting Salmonella infection.
Keywords: Salmonella; gut microbiota; host metabolism during infection; host-microbe interaction; microbial metabolism.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
LACTATEing Salmonella: A Host-Derived Fermentation Product Fuels Pathogen Growth.Cell Host Microbe. 2018 Jan 10;23(1):3-4. doi: 10.1016/j.chom.2017.12.012. Cell Host Microbe. 2018. PMID: 29324228
References
-
- Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, Madison BB, Gumucio DL, Marin HE, Peters JM, Young HA, et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55(8):1104–1113. doi: 10.1136/gut.2005.081745. Published online 2006/03/21. - DOI - PMC - PubMed
-
- Ali MM, Newsom DL, Gonzalez JF, Sabag-Daigle A, Stahl C, Steidley B, Dubena J, Dyszel JL, Smith JN, Dieye Y, et al. Fructose-asparagine is a primary nutrient during growth of Salmonella in the inflamed intestine. PLoS Pathog. 2014;10(6):e1004209. doi: 10.1371/journal.ppat.1004209. Published online 2014/06/27. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

