Guided bone regeneration with asymmetric collagen-chitosan membranes containing aspirin-loaded chitosan nanoparticles
- PMID: 29276386
- PMCID: PMC5733920
- DOI: 10.2147/IJN.S148179
Guided bone regeneration with asymmetric collagen-chitosan membranes containing aspirin-loaded chitosan nanoparticles
Abstract
Introduction: Membranes allowing the sustained release of drugs that can achieve cell adhesion are very promising for guided bone regeneration. Previous studies have suggested that aspirin has the potential to promote bone regeneration. The purpose of this study was to prepare a local drug delivery system with aspirin-loaded chitosan nanoparticles (ACS) contained in an asymmetric collagen-chitosan membrane (CCM).
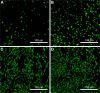
Methods: In this study, the ACS were fabricated using different concentrations of aspirin (5 mg, 25 mg, 50 mg, and 75 mg). The drug release behavior of ACS was studied. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were used to examine the micromorphology of ACS and aspirin-loaded chitosan nanoparticles contained in chitosan-collagen membranes (ACS-CCM). In vitro bone mesenchymal stem cells (BMSCs) were cultured and critical-sized cranial defects on Sprague-Dawley rats were made to evaluate the effect of the ACS-CCM on bone regeneration.
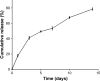
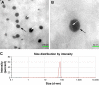
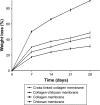
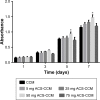
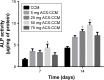
Results: Drug release behavior results of ACS showed that the nanoparticles fabricated in this study could successfully sustain the release of the drug. TEM showed the morphology of the nanoparticles. SEM images indicated that the asymmetric membrane comprised a loose collagen layer and a dense chitosan layer. In vitro studies showed that ACS-CCM could promote the proliferation of BMSCs, and that the degree of differentiated BMSCs seeded on CCMs containing 50 mg of ACS was higher than that of other membranes. Micro-computed tomography showed that 50 mg of ACS-CCM resulted in enhanced bone regeneration compared with the control group.
Conclusion: This study shows that the ACS-CCM would allow the sustained release of aspirin and have further osteogenic potential. This membrane is a promising therapeutic approach to guiding bone regeneration.
Keywords: aspirin; drug release; guided bone regeneration; membrane; nanoparticle.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures




References
-
- Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31(31):7892–7927. - PubMed
-
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. - PubMed
-
- Bashutski JD, Wang HL. Periodontal and endodontic regeneration. J Endod. 2009;35(3):321–328. - PubMed
-
- Zhang K, Zhao M, Cai L, Wang Zk, Sun Yf, Hu QL. Preparation of chitosan/hydroxyapatite guided membrane used for periodontal tissue regeneration. Chinese Journal of Polymer Science. 2010;28(4):555–561.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

