Experimental Gene Therapy with Serine-Histogranin and Endomorphin 1 for the Treatment of Chronic Neuropathic Pain
- PMID: 29276474
- PMCID: PMC5727090
- DOI: 10.3389/fnmol.2017.00406
Experimental Gene Therapy with Serine-Histogranin and Endomorphin 1 for the Treatment of Chronic Neuropathic Pain
Abstract
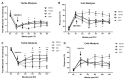
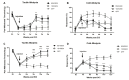
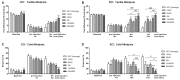
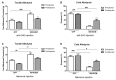
The insufficient pain relief provided by current pharmacotherapy for chronic neuropathic pain is a serious medical problem. The enhanced glutamate signaling via NMDA receptors appears to be one of the key events in the development of chronic pain. Although effective, clinical use of systemic NMDA antagonists is limited by adverse effects such as hallucinations and motor dysfunction. Opioids are also potent analgesics but their chronic use is accompanied by tolerance and risk of addiction. However, combination of NMDA antagonists and opioids seems to provide a stable pain relieve at subthreshold doses of both substances, eliminating development of side effects. Our previous research showed that combined delivery of NMDA antagonist Serine histrogranin (SHG) and endomorphin1 (EM1) leads to attenuation of acute and chronic pain. The aim of this study was to design and evaluate an analgesic potency of the gene construct encoding SHG and EM1. Constructs with 1SHG copy in combination with EM1, 1SHG/EM1, and 6SHG/EM1 were intraspinally injected to animals with peripheral nerve injury-induced pain (chronic constriction injury, CCI) or spinal cord injury induced pain (clip compression model, SCI) and tactile and cold allodynia were evaluated. AAV2/8 particles were used for gene delivery. The results demonstrated 6SHG/EM1 as the most efficient for alleviation of pain-related behavior. The effect was observed up to 8 weeks in SCI animals, suggesting the lack of tolerance of possible synergistic effect between SHG and EM1. Intrathecal injection of SHG antibody or naloxone attenuated the analgesic effect in treated animals. Biochemical and histochemical evaluation confirmed the presence of both peptides in the spinal tissue. The results of this study showed that the injection of AAV vectors encoding combined SHG/EM constructs can provide long term attenuation of pain without overt adverse side effects. This approach may provide better treatment options for patients suffering from chronic pain.
Keywords: endomorphin 1; gene therapy; nerve injury; neuropathic pain; serine histogranin; spinal cord injury.
Figures


References
-
- Beutler A. S., Banck M. S., Walsh C. E., Milligan E. D. (2005). Intrathecal gene transfer by adeno-associated virus for pain. Curr. Opin. Mol. Ther. 7, 431–439. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

