Critical Points and Traveling Wave in Locomotion: Experimental Evidence and Some Theoretical Considerations
- PMID: 29276476
- PMCID: PMC5727018
- DOI: 10.3389/fncir.2017.00098
Critical Points and Traveling Wave in Locomotion: Experimental Evidence and Some Theoretical Considerations
Abstract
The central pattern generator (CPG) architecture for rhythm generation remains partly elusive. We compare cat and frog locomotion results, where the component unrelated to pattern formation appears as a temporal grid, and traveling wave respectively. Frog spinal cord microstimulation with N-methyl-D-Aspartate (NMDA), a CPG activator, produced a limited set of force directions, sometimes tonic, but more often alternating between directions similar to the tonic forces. The tonic forces were topographically organized, and sites evoking rhythms with different force subsets were located close to the constituent tonic force regions. Thus CPGs consist of topographically organized modules. Modularity was also identified as a limited set of muscle synergies whose combinations reconstructed the EMGs. The cat CPG was investigated using proprioceptive inputs during fictive locomotion. Critical points identified both as abrupt transitions in the effect of phasic perturbations, and burst shape transitions, had biomechanical correlates in intact locomotion. During tonic proprioceptive perturbations, discrete shifts between these critical points explained the burst durations changes, and amplitude changes occurred at one of these points. Besides confirming CPG modularity, these results suggest a fixed temporal grid of anchoring points, to shift modules onsets and offsets. Frog locomotion, reconstructed with the NMDA synergies, showed a partially overlapping synergy activation sequence. Using the early synergy output evoked by NMDA at different spinal sites, revealed a rostrocaudal topographic organization, where each synergy is preferentially evoked from a few, albeit overlapping, cord regions. Comparing the locomotor synergy sequence with this topography suggests that a rostrocaudal traveling wave would activate the synergies in the proper sequence for locomotion. This output was reproduced in a two-layer model using this topography and a traveling wave. Together our results suggest two CPG components: modules, i.e., synergies; and temporal patterning, seen as a temporal grid in the cat, and a traveling wave in the frog. Animal and limb navigation have similarities. Research relating grid cells to the theta rhythm and on segmentation during navigation may relate to our temporal grid and traveling wave results. Winfree's mathematical work, combining critical phases and a traveling wave, also appears important. We conclude suggesting tracing, and imaging experiments to investigate our CPG model.
Keywords: Winfree’s phase singularities; central pattern generator; critical point shifts; hippocampus; locomotion; spinal cord; temporal grid; traveling wave.
Figures

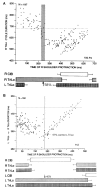




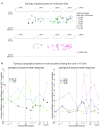
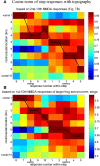

Similar articles
-
Synergy temporal sequences and topography in the spinal cord: evidence for a traveling wave in frog locomotion.Brain Struct Funct. 2016 Nov;221(8):3869-3890. doi: 10.1007/s00429-015-1133-5. Epub 2015 Oct 26. Brain Struct Funct. 2016. PMID: 26501407
-
Motor module activation sequence and topography in the spinal cord during air-stepping in human: Insights into the traveling wave in spinal locomotor circuits.Physiol Rep. 2017 Nov;5(22):e13504. doi: 10.14814/phy2.13504. Physiol Rep. 2017. PMID: 29180480 Free PMC article.
-
Regional distribution of putative rhythm-generating and pattern-forming components of the mammalian locomotor CPG.Neuroscience. 2013 Oct 10;250:644-50. doi: 10.1016/j.neuroscience.2013.07.070. Epub 2013 Aug 8. Neuroscience. 2013. PMID: 23933310
-
The Human Central Pattern Generator for Locomotion: Does It Exist and Contribute to Walking?Neuroscientist. 2017 Dec;23(6):649-663. doi: 10.1177/1073858417699790. Epub 2017 Mar 28. Neuroscientist. 2017. PMID: 28351197 Review.
-
Chapter 2--the spinal generation of phases and cycle duration.Prog Brain Res. 2011;188:15-29. doi: 10.1016/B978-0-444-53825-3.00007-3. Prog Brain Res. 2011. PMID: 21333800 Review.
Cited by
-
Gradient descent decomposition of force-field motor primitives optogenetically elicited for motor mapping of the murine lumbosacral spinal cord.Zool Res. 2023 May 18;44(3):604-619. doi: 10.24272/j.issn.2095-8137.2022.276. Zool Res. 2023. PMID: 36785931 Free PMC article.
-
Towards an Understanding of Control of Complex Rhythmical "Wavelike" Coordination in Humans.Brain Sci. 2020 Apr 5;10(4):215. doi: 10.3390/brainsci10040215. Brain Sci. 2020. PMID: 32260547 Free PMC article. Review.
-
Why Firing Rate Distributions Are Important for Understanding Spinal Central Pattern Generators.Front Hum Neurosci. 2021 Sep 3;15:719388. doi: 10.3389/fnhum.2021.719388. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34539363 Free PMC article.
-
Intersegmental Interactions Give Rise to a Global Network.Front Neural Circuits. 2022 Feb 23;16:843731. doi: 10.3389/fncir.2022.843731. eCollection 2022. Front Neural Circuits. 2022. PMID: 35282329 Free PMC article.
-
Adaptive hindlimb split-belt treadmill walking in rats by controlling basic muscle activation patterns via phase resetting.Sci Rep. 2018 Nov 26;8(1):17341. doi: 10.1038/s41598-018-35714-8. Sci Rep. 2018. PMID: 30478405 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

