Host and Pathway Engineering for Enhanced Lycopene Biosynthesis in Yarrowia lipolytica
- PMID: 29276501
- PMCID: PMC5727423
- DOI: 10.3389/fmicb.2017.02233
Host and Pathway Engineering for Enhanced Lycopene Biosynthesis in Yarrowia lipolytica
Abstract
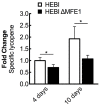
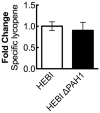
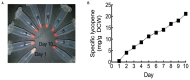
Carotenoids are a class of molecules with commercial value as food and feed additives with nutraceutical properties. Shifting carotenoid synthesis from petrochemical-based precursors to bioproduction from sugars and other biorenewable carbon sources promises to improve process sustainability and economics. In this work, we engineered the oleaginous yeast Yarrowia lipolytica to produce the carotenoid lycopene. To enhance lycopene production, we tested a series of strategies to modify host cell physiology and metabolism, the most successful of which were mevalonate pathway overexpression and alleviating auxotrophies previously engineered into the PO1f strain of Y. lipolytica. The beneficial engineering strategies were combined into a single strain, which was then cultured in a 1-L bioreactor to produce 21.1 mg/g DCW. The optimized strain overexpressed a total of eight genes including two copies of HMG1, two copies of CrtI, and single copies of MVD1, EGR8, CrtB, and CrtE. Recovering leucine and uracil biosynthetic capacity also produced significant enhancement in lycopene titer. The successful engineering strategies characterized in this work represent a significant increase in understanding carotenoid biosynthesis in Y. lipolytica, not only increasing lycopene titer but also informing future studies on carotenoid biosynthesis.
Keywords: HMG1; carotenoids; lipid metabolism; metabolic engineering; mevalonate pathway; synthetic biology.
Figures







Similar articles
-
Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica.Appl Environ Microbiol. 2014 Mar;80(5):1660-9. doi: 10.1128/AEM.03167-13. Epub 2013 Dec 27. Appl Environ Microbiol. 2014. PMID: 24375130 Free PMC article.
-
Iterative gene integration mediated by 26S rDNA and non-homologous end joining for the efficient production of lycopene in Yarrowia lipolytica.Bioresour Bioprocess. 2023 Nov 24;10(1):83. doi: 10.1186/s40643-023-00697-6. Bioresour Bioprocess. 2023. PMID: 38647953 Free PMC article.
-
Bioengineering of oleaginous yeast Yarrowia lipolytica for lycopene production.Methods Mol Biol. 2012;898:153-9. doi: 10.1007/978-1-61779-918-1_9. Methods Mol Biol. 2012. PMID: 22711123
-
Biotechnological Production of Carotenoids Using Yarrowia lipolytica.J Agric Food Chem. 2025 Mar 26;73(12):7034-7045. doi: 10.1021/acs.jafc.4c11251. Epub 2025 Mar 13. J Agric Food Chem. 2025. PMID: 40079666 Review.
-
Advances in synthetic biology of oleaginous yeast Yarrowia lipolytica for producing non-native chemicals.Appl Microbiol Biotechnol. 2018 Jul;102(14):5925-5938. doi: 10.1007/s00253-018-9099-x. Epub 2018 May 28. Appl Microbiol Biotechnol. 2018. PMID: 29808327 Review.
Cited by
-
PASIV: A Pooled Approach-Based Workflow to Overcome Toxicity-Induced Design of Experiments Failures and Inefficiencies.ACS Synth Biol. 2022 Mar 18;11(3):1272-1291. doi: 10.1021/acssynbio.1c00562. Epub 2022 Mar 9. ACS Synth Biol. 2022. PMID: 35261238 Free PMC article.
-
Yarrowia lipolytica Strains Engineered for the Production of Terpenoids.Front Bioeng Biotechnol. 2020 Aug 14;8:945. doi: 10.3389/fbioe.2020.00945. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32923433 Free PMC article.
-
Elevated β-Carotene Production Using Codon-Adapted CarRA&B and Metabolic Balance in Engineered Yarrowia lipolytica.Front Microbiol. 2021 Mar 4;12:627150. doi: 10.3389/fmicb.2021.627150. eCollection 2021. Front Microbiol. 2021. PMID: 33746920 Free PMC article.
-
Optimization of microbial cell factories for astaxanthin production: Biosynthesis and regulations, engineering strategies and fermentation optimization strategies.Synth Syst Biotechnol. 2022 Feb 18;7(2):689-704. doi: 10.1016/j.synbio.2022.01.002. eCollection 2022 Jun. Synth Syst Biotechnol. 2022. PMID: 35261927 Free PMC article. Review.
-
Changing Form and Function through Carotenoids and Synthetic Biology.Plant Physiol. 2019 Mar;179(3):830-843. doi: 10.1104/pp.18.01122. Epub 2018 Oct 25. Plant Physiol. 2019. PMID: 30361256 Free PMC article.
References
-
- Albrecht M., Misawa N., Sandmann G. (1999). Metabolic engineering of the terpenoid biosynthetic pathway of Escherichia coli for production of the carotenoids beta-carotene and zeaxanthin. Biotechnol. Lett. 21 791–795. 10.1023/A:1005547827380 - DOI
-
- Armstrong G. A., Hearst J. E. (1996). Carotenoids.2. Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 10 228–237. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

