Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms
- PMID: 29276503
- PMCID: PMC5727068
- DOI: 10.3389/fmicb.2017.02407
Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms
Abstract
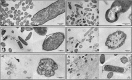
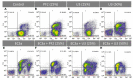
Chronic wounds afford a hostile environment of damaged tissues that allow bacterial proliferation and further wound colonization. Escherichia coli is among the most common colonizers of infected wounds and it is a prolific biofilm former. Living in biofilm communities, cells are protected, become more difficult to control and eradicate, and less susceptible to antibiotic therapy. This work presents insights into the proceedings triggering E. coli biofilm control with phage, honey, and their combination, achieved through standard antimicrobial activity assays, zeta potential and flow cytometry studies and further visual insights sought by scanning electron microscopy and transmission electron microscopy. Two Portuguese honeys (PF2 and U3) with different floral origin and an E. coli-specific phage (EC3a), possessing depolymerase activity, were tested against 24- and 48-h-old biofilms. Synergic and additive effects were perceived in some phage-honey experiments. Combined therapy prompted similar phenomena in biofilm cells, visualized by electron microscopy, as the individual treatments. Honey caused minor membrane perturbations to complete collapse and consequent discharge of cytoplasmic content, and phage completely destroyed cells leaving only vesicle-like structures and debris. Our experiments show that the addition of phage to low honey concentrations is advantageous, and that even fourfold diluted honey combined with phage, presents no loss of antibacterial activity toward E. coli. Portuguese honeys possess excellent antibiofilm activity and may be potential alternative therapeutic agents in biofilm-related wound infection. Furthermore, to our knowledge this is the first study that assessed the impacts of phage-honey combinations in bacterial cells. The synergistic effect obtained was shown to be promising, since the antiviral effect of honey limits the emergence of phage resistant phenotypes.
Keywords: E. coli; bacteriophage; biofilms; honey; synergy.
Figures

Similar articles
-
Chestnut Honey and Bacteriophage Application to Control Pseudomonas aeruginosa and Escherichia coli Biofilms: Evaluation in an ex vivo Wound Model.Front Microbiol. 2018 Jul 31;9:1725. doi: 10.3389/fmicb.2018.01725. eCollection 2018. Front Microbiol. 2018. PMID: 30108574 Free PMC article.
-
Manuka-type honeys can eradicate biofilms produced by Staphylococcus aureus strains with different biofilm-forming abilities.PeerJ. 2014 Mar 25;2:e326. doi: 10.7717/peerj.326. eCollection 2014. PeerJ. 2014. PMID: 24711974 Free PMC article.
-
Rifampicin-Manuka Honey Combinations Are Superior to Other Antibiotic-Manuka Honey Combinations in Eradicating Staphylococcus aureus Biofilms.Front Microbiol. 2018 Jan 11;8:2653. doi: 10.3389/fmicb.2017.02653. eCollection 2017. Front Microbiol. 2018. PMID: 29375518 Free PMC article.
-
Use of an engineered honey to eradicate preformed biofilms of important wound pathogens: an in vitro study.J Wound Care. 2017 Aug 2;26(8):442-450. doi: 10.12968/jowc.2017.26.8.442. J Wound Care. 2017. PMID: 28795889
-
Sweet and sour synergy: exploring the antibacterial and antibiofilm activity of acetic acid and vinegar combined with medical-grade honeys.Microbiology (Reading). 2023 Jul;169(7):001351. doi: 10.1099/mic.0.001351. Microbiology (Reading). 2023. PMID: 37435775 Free PMC article.
Cited by
-
Phage-Host Interaction Analysis by Flow Cytometry Allows for Rapid and Efficient Screening of Phages.Antibiotics (Basel). 2022 Jan 27;11(2):164. doi: 10.3390/antibiotics11020164. Antibiotics (Basel). 2022. PMID: 35203767 Free PMC article.
-
Pitfalls Associated with Discriminating Mixed-Species Biofilms by Flow Cytometry.Antibiotics (Basel). 2020 Oct 27;9(11):741. doi: 10.3390/antibiotics9110741. Antibiotics (Basel). 2020. PMID: 33121057 Free PMC article.
-
Honey Combination Therapies for Skin and Wound Infections: A Systematic Review of the Literature.Clin Cosmet Investig Dermatol. 2020 Nov 24;13:875-888. doi: 10.2147/CCID.S282143. eCollection 2020. Clin Cosmet Investig Dermatol. 2020. PMID: 33262630 Free PMC article. Review.
-
New Strategies to Kill Metabolically-Dormant Cells Directly Bypassing the Need for Active Cellular Processes.Antibiotics (Basel). 2023 Jun 12;12(6):1044. doi: 10.3390/antibiotics12061044. Antibiotics (Basel). 2023. PMID: 37370363 Free PMC article. Review.
-
Encapsulation of E. coli phage ZCEC5 in chitosan-alginate beads as a delivery system in phage therapy.AMB Express. 2019 Jun 17;9(1):87. doi: 10.1186/s13568-019-0810-9. AMB Express. 2019. PMID: 31209685 Free PMC article.
References
-
- Adams M. H. (1959). Bacteriophages. New York, NY: Interscience Publishers.
LinkOut - more resources
Full Text Sources
Other Literature Sources

