Anti-biofilm Properties of Bacterial Di-Rhamnolipids and Their Semi-Synthetic Amide Derivatives
- PMID: 29276509
- PMCID: PMC5727045
- DOI: 10.3389/fmicb.2017.02454
Anti-biofilm Properties of Bacterial Di-Rhamnolipids and Their Semi-Synthetic Amide Derivatives
Abstract
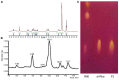
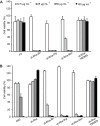
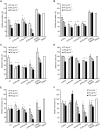
A new strain, namely Lysinibacillus sp. BV152.1 was isolated from the rhizosphere of ground ivy (Glechoma hederacea L.) producing metabolites with potent ability to inhibit biofilm formation of an important human pathogens Pseudomonas aeruginosa PAO1, Staphylococcus aureus, and Serratia marcescens. Structural characterization revealed di-rhamnolipids mixture containing rhamnose (Rha)-Rha-C10-C10, Rha-Rha-C8-C10, and Rha-Rha-C10-C12 in the ratio 7:2:1 as the active principle. Purified di-rhamnolipids, as well as commercially available di-rhamnolipids (Rha-Rha-C10-C10, 93%) were used as the substrate for the chemical derivatization for the first time, yielding three semi-synthetic amide derivatives, benzyl-, piperidine-, and morpholine. A comparative study of the anti-biofilm, antibacterial and cytotoxic properties revealed that di-Rha from Lysinibacillus sp. BV152.1 were more potent in biofilm inhibition, both cell adhesion and biofilm maturation, than commercial di-rhamnolipids inhibiting 50% of P. aeruginosa PAO1 biofilm formation at 50 μg mL-1 and 75 μg mL-1, respectively. None of the di-rhamnolipids exhibited antimicrobial properties at concentrations of up to 500 μg mL-1. Amide derivatization improved inhibition of biofilm formation and dispersion activities of di-rhamnolipids from both sources, with morpholine derivative being the most active causing more than 80% biofilm inhibition at concentrations 100 μg mL-1. Semi-synthetic amide derivatives showed increased antibacterial activity against S. aureus, and also showed higher cytotoxicity. Therefore, described di-rhamnolipids are potent anti-biofilm agents and the described approach can be seen as viable approach in reaching new rhamnolipid based derivatives with tailored biological properties.
Keywords: amide derivative; biofilms; cell adhesion; di-rhamnolipids; rhamnolipids.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

