Recent Successes and Future Directions in Immunotherapy of Cutaneous Melanoma
- PMID: 29276510
- PMCID: PMC5727014
- DOI: 10.3389/fimmu.2017.01617
Recent Successes and Future Directions in Immunotherapy of Cutaneous Melanoma
Abstract
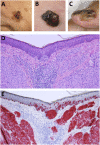
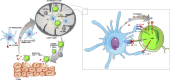
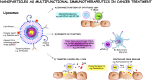
The global health burden associated with melanoma continues to increase while treatment options for metastatic melanoma are limited. Nevertheless, in the past decade, the field of cancer immunotherapy has witnessed remarkable advances for the treatment of a number of malignancies including metastatic melanoma. Although the earliest observations of an immunological antitumor response were made nearly a century ago, it was only in the past 30 years, that immunotherapy emerged as a viable therapeutic option, in particular for cutaneous melanoma. As such, melanoma remains the focus of various preclinical and clinical studies to understand the immunobiology of cancer and to test various tumor immunotherapies. Here, we review key recent developments in the field of immune-mediated therapy of melanoma. Our primary focus is on therapies that have received regulatory approval. Thus, a brief overview of the pathophysiology of melanoma is provided. The purported functions of various tumor-infiltrating immune cell subsets are described, in particular the recently described roles of intratumoral dendritic cells. The section on immunotherapies focuses on strategies that have proved to be the most clinically successful such as immune checkpoint blockade. Prospects for novel therapeutics and the potential for combinatorial approaches are delineated. Finally, we briefly discuss nanotechnology-based platforms which can in theory, activate multiple arms of immune system to fight cancer. The promising advances in the field of immunotherapy signal the dawn of a new era in cancer treatment and warrant further investigation to understand the opportunities and barriers for future progress.
Keywords: adoptive T cell transfer; immune checkpoint blockade; immunotherapy; melanoma; programmed cell death protein 1; tumor microenvironment; tumor-infiltrating dendritic cell; tumor-infiltrating lymphocyte.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

