Low-Density Lipoprotein Receptor Deficiency Attenuates Neuroinflammation through the Induction of Apolipoprotein E
- PMID: 29276512
- PMCID: PMC5727422
- DOI: 10.3389/fimmu.2017.01701
Low-Density Lipoprotein Receptor Deficiency Attenuates Neuroinflammation through the Induction of Apolipoprotein E
Abstract
Objective: We aimed to determine the role of the low-density lipoprotein receptor (LDLr) in neuroinflammation by inducing experimental autoimmune encephalomyelitis (EAE) in ldlr knock out mice.
Methods: MOG35-55 induced EAE in male and female ldlr-/- mice was assessed clinically and histopathologically. Expression of inflammatory mediators and apolipoprotein E (apoE) was investigated by qPCR. Changes in protein levels of apoE and tumor necrosis factor alpha (TNFα) were validated by western blot and ELISA, respectively.
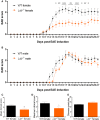
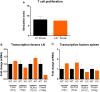
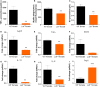
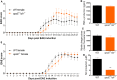
Results: Ldlr-/--attenuated EAE disease severity in female, but not in male, EAE mice marked by a reduced proinflammatory cytokine production in the central nervous system of female ldlr-/- mice. Macrophages from female ldlr-/- mice showed a similar decrease in proinflammatory mediators, an impaired capacity to phagocytose myelin and enhanced secretion of the anti-inflammatory apoE. Interestingly, apoE/ldlr double knock out abrogated the beneficial effect of ldlr depletion in EAE.
Conclusion: Collectively, we show that ldlr-/- reduces EAE disease severity in female but not in male EAE mice, and that this can be explained by increased levels of apoE in female ldlr-/- mice. Although the reason for the observed sexual dimorphism remains unclear, our findings show that LDLr and associated apoE levels are involved in neuroinflammatory processes.
Keywords: apolipoprotein E; experimental autoimmune encephalomyelitis; low-density lipoprotein receptor; multiple sclerosis; neuroinflammation.
Figures



References
-
- Brosnan CF, Bornstein MB, Bloom BR. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J Immunol (1981) 126(2):614–20. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

