Microencapsulation of reuterin to enhance long-term efficacy against food-borne pathogen Listeria monocytogenes
- PMID: 29276661
- PMCID: PMC5735039
- DOI: 10.1007/s13205-017-1035-8
Microencapsulation of reuterin to enhance long-term efficacy against food-borne pathogen Listeria monocytogenes
Abstract
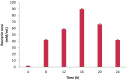
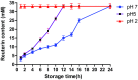
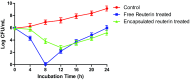
The aim of this study was to microencapsulate the reuterin produced by Lactobacillus reuteri BPL-36 strain for its long-term efficacy against food-borne pathogen Listeria monocytogenes. Lactobacillus reuteri BPL-36 strain previously isolated from a human infant fecal sample in lab was selected for the present study based on its ability to produce reuterin. The organism displayed a broad-spectrum antimicrobial activity. Reuterin concentration of 89.63 mM was obtained in the MRS-glycerol medium after 16 h incubation at 37 °C. The reuterin concentration required to inhibit the growth of Pseudomonas aeruginosa, Escherichia coli O157: H7, Salmonella typhi, Staphylococcus aureus, and Listeria monocytogenes was found to be 1.0, 2.0, 2.0, 4.0, and 10.0 AU/mL, respectively. Microencapsulation of reuterin to enhance long-term efficacy against food-borne pathogens was done. Results in this study indicated that the release characteristics of reuterin from the encapsulated particles were pH dependent. The release characteristics were unaffected by the storage of encapsulated reuterin at 4 °C for 2 weeks. The anti-listerial efficacy of the encapsulated reuterin was tested against L. monocytogenes in the BHI medium adjusted to pH 5.0 with a reuterin content equivalent to 16 mM, similar to un-encapsulated (free) reuterin. Encapsulated reuterin demonstrated enhanced efficacy against L. monocytogenes for longer duration of time when compared with un-encapsulated (free) reuterin. The present work demonstrated a novel antimicrobial delivery system that ensured much better capability of inhibiting the growth of L. monocytogenes throughout 24 h incubation at 37 °C.
Keywords: Lactobacillus reuteri; Microencapsulation; Pathogens; Reuterin; Safety.
Conflict of interest statement
Compliance with ethical standardsThis article does not contain any studies with human or animal subjects.Additional informed consents were obtained from all the parents of infants from whom fecal samples were collected for isolation of lactic acid bacteria.Santosh Kumar Mishra, R. K. Malik, Harsh Panwar, and Amit Kumar Barui declare that they have no conflict of interest.
Figures


Similar articles
-
Characterization of a Reuterin-Producing Lactobacillus reuteri BPL-36 Strain Isolated from Human Infant Fecal Sample.Probiotics Antimicrob Proteins. 2012 Sep;4(3):154-61. doi: 10.1007/s12602-012-9103-1. Probiotics Antimicrob Proteins. 2012. PMID: 26782041
-
Application of reuterin produced by Lactobacillus reuteri 12002 for meat decontamination and preservation.J Food Prot. 1999 Mar;62(3):257-61. doi: 10.4315/0362-028x-62.3.257. J Food Prot. 1999. PMID: 10090245
-
The effect of Reuterin on Listeria monocytogenes and Escherichia coli O157:H7 in milk and cottage cheese.J Food Prot. 1998 Oct;61(10):1275-80. doi: 10.4315/0362-028x-61.10.1275. J Food Prot. 1998. PMID: 9798141
-
Short communication: Combined antimicrobial activity of reuterin and diacetyl against foodborne pathogens.J Dairy Sci. 2014 Oct;97(10):6116-21. doi: 10.3168/jds.2014-8306. Epub 2014 Jul 30. J Dairy Sci. 2014. PMID: 25087026
-
Antimicrobial activity of reuterin produced by Lactobacillus reuteri on Listeria monocytogenes in cold-smoked salmon.Food Microbiol. 2014 Dec;44:1-5. doi: 10.1016/j.fm.2014.05.006. Epub 2014 May 22. Food Microbiol. 2014. PMID: 25084638
Cited by
-
Continuous-Flow Separation and Efficient Concentration of Foodborne Bacteria from Large Volume Using Nickel Nanowire Bridge in Microfluidic Chip.Micromachines (Basel). 2019 Sep 25;10(10):644. doi: 10.3390/mi10100644. Micromachines (Basel). 2019. PMID: 31557924 Free PMC article.
-
Effective encapsulation of reuterin-producing Limosilactobacillus reuteri in alginate beads prepared with different mucilages/gums.Biotechnol Rep (Amst). 2022 May 14;34:e00737. doi: 10.1016/j.btre.2022.e00737. eCollection 2022 Jun. Biotechnol Rep (Amst). 2022. PMID: 35686007 Free PMC article.
-
Eco-Friendly Edible Packaging Systems Based on Live-Lactobacillus kefiri MM5 for the Control of Listeria monocytogenes in Fresh Vegetables.Foods. 2022 Aug 30;11(17):2632. doi: 10.3390/foods11172632. Foods. 2022. PMID: 36076818 Free PMC article.
References
-
- Axelsson LT, Chung TC, Dobrogosz WJ, Lindgren SE. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb Ecol Health Dis. 1989;2:131–136. doi: 10.3109/08910608909140210. - DOI
-
- Chung TC, Axelsson L, Lindgren SE, Dobrogosz WJ. In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microb Eco Health Dis. 1989;2(2):137–144.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

