Characterization of Ixodes ricinus Fibrinogen-Related Proteins (Ixoderins) Discloses Their Function in the Tick Innate Immunity
- PMID: 29276701
- PMCID: PMC5727070
- DOI: 10.3389/fcimb.2017.00509
Characterization of Ixodes ricinus Fibrinogen-Related Proteins (Ixoderins) Discloses Their Function in the Tick Innate Immunity
Abstract
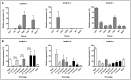
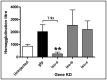
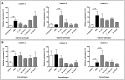
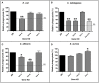
Ticks are important vectors of serious human and animal disease-causing organisms, but their innate immunity can fight invading pathogens and may have the ability to reduce or block transmission to mammalian hosts. Lectins, sugar-binding proteins, can distinguish between self and non-self-oligosaccharide motifs on pathogen surfaces. Although tick hemolymph possesses strong lectin activity, and several lectins have already been isolated and characterized, little is known about the implementation of these molecules in tick immunity. Here, we have described and functionally characterized fibrinogen-related protein (FReP) lectins in Ixodes ticks. We have shown that the FReP family contains at least 27 genes (ixoderins, ixo) that could, based on phylogenetic and expression analyses, be divided into three groups with differing degrees of expansion. By using RNA interference-mediated gene silencing (RNAi) we demonstrated that IXO-A was the main lectin in tick hemolymph. Further, we found that ixoderins were important for phagocytosis of Gram-negative bacteria and yeasts by tick hemocytes and that their expression was upregulated upon injection of microbes, wounding, or after blood feeding. However, although the tick hemocytes could swiftly phagocytose Borrelia afzelii spirochetes, their transmission and burst of infection in mice was not altered. Our results demonstrate that tick ixoderins are crucial immune proteins that work as opsonins in the tick hemolymph, targeting microbes for phagocytosis or lysis.
Keywords: Borrelia; Ixodes; RNAi; complement; fibrinogen-related protein; ixoderin; lectin; tick.
Figures

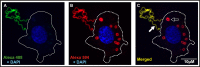

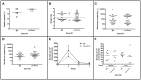
References
-
- DeFranco A., Locksley R. M., Robertson M. (2007). Immunity: The Immune Response in Infectious and Inflammatory Disease. London: New Science Press.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

