Embryonic exposure to Mono(2-ethylhexyl) phthalate (MEHP) disrupts pancreatic organogenesis in zebrafish (Danio rerio)
- PMID: 29277029
- PMCID: PMC5788038
- DOI: 10.1016/j.chemosphere.2017.12.094
Embryonic exposure to Mono(2-ethylhexyl) phthalate (MEHP) disrupts pancreatic organogenesis in zebrafish (Danio rerio)
Abstract
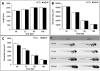
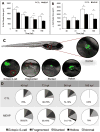
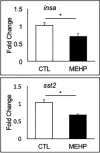
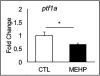
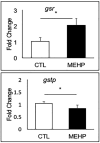
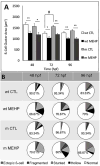
Mono(2-ethylhexyl) phthalate (MEHP) is the bioactive metabolite of di(2-ethylhexyl) phthalate, a plasticizing agent and persistent environmental contaminant associated with obesity, developmental abnormalities, and oxidative stress. Nrf2 (Nfe2l2) is a transcription factor that regulates cytoprotective genes as part of the adaptive antioxidant response. We previously identified the pancreas as a sensitive target of oxidative stress during embryonic development. The goals of this study were to 1) characterize the effects of MEHP exposure on pancreatic development, and 2) determine whether oxidative stress contributes to MEHP embryotoxicity. Zebrafish (Danio rerio) embryos from AB wildtype and Tg(ins:GFP;nrf2afh318/fh318) were exposed to 0 or 200 μg/L MEHP at 3 h post fertilization (hpf) through 168 hpf to assess pancreatic organogenesis. MEHP exposure significantly decreased β-cell area at all timepoints (48, 72, 96, 168 hpf), but Nrf2a did not significantly protect against islet hypomorphism. Tg(gcga:GFP) embryos exposed to MEHP showed a decrease in α-cell area in the islet across the same timepoints. Tg(ptf1a:GFP) embryos were assessed at 80 and 168 hpf for exocrine pancreas length. MEHP exposure decreased growth of the exocrine pancreas. Expression of pancreas genes insa, sst2 and ptf1a was significantly reduced by MEHP exposure compared to controls. Glutathione (GSH) concentrations and redox potentials were quantified at 72 hpf by HPLC, but no significant changes were observed. However, expression of the GSH-related genes gstp1 and gsr were significantly altered by MEHP exposure. These data indicate that the developing pancreas is a sensitive target tissue of embryonic exposure to MEHP.
Keywords: Endocrine; Exocrine; Islet; Nfe2l2; Redox; Toxicology.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

References
-
- Agabi JO, Akhigbe AO. Comparative sonographic evaluation of the anteroposterior dimensions of the pancreas in diabetics and nondiabetics. Niger J Clin Pract. 2016;19:175–81. - PubMed
-
- Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J Hazard Mater. 2017;340:360–383. - PubMed
-
- Bento A, Baptista H, Oliveira F. Congenital pancreas malformations: a clinical case report. Rev Assoc Med Bras (1992) 2013;59:35–9. - PubMed
-
- Corbasson I, Hankinson SE, Stanek EJ, Reeves KW., 3 Urinary bisphenol-A, phthalate metabolites and body composition in US adults, NHANES 1999–2006. Int J Environ Health Res. 2016;26:606–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

