Nuclear Receptor Subfamily 1 Group D Member 1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce the Severity of Fulminant Hepatitis in Mice
- PMID: 29277561
- PMCID: PMC5892845
- DOI: 10.1053/j.gastro.2017.12.019
Nuclear Receptor Subfamily 1 Group D Member 1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce the Severity of Fulminant Hepatitis in Mice
Abstract
Background & aims: The innate immune system responds not only to bacterial signals, but also to non-infectious danger-associated molecular patterns that activate the NLRP3 inflammasome complex after tissue injury. Immune functions vary over the course of the day, but it is not clear whether these changes affect the activity of the NLRP3 inflammasome. We investigated whether the core clock component nuclear receptor subfamily 1 group D member 1 (NR1D1, also called Rev-erbα) regulates expression, activity of the NLRP3 inflammasome, and its signaling pathway.
Methods: We collected naïve peritoneal macrophages and plasma, at multiple times of day, from Nr1d1-/- mice and their Nr1d1+/+ littermates (controls) and analyzed expression NLRP3, interleukin 1β (IL1B, in plasma), and IL18 (in plasma). We also collected bone marrow-derived primary macrophages from these mice. Levels of NR1D1 were knocked down with small hairpin RNAs in human primary macrophages. Bone marrow-derived primary macrophages from mice and human primary macrophages were incubated with lipopolysaccharide (LPS) to induce expression of NLRP3, IL1B, and IL18; cells were incubated with LPS and adenosine triphosphate to activate the NLRP3 complex. We analyzed caspase 1 activity and cytokine secretion. NR1D1 was activated in primary mouse and human macrophages by incubation with SR9009; some of the cells were also incubated with an NLRP3 inhibitor or inhibitors of caspase 1. Nr1d1-/- mice and control mice were given intraperitoneal injections of LPS to induce peritoneal inflammation; plasma samples were isolated and levels of cytokines were measured. Nr1d1-/- mice, control mice, and control mice given injections of SR9009 were given LPS and D-galactosamine to induce fulminant hepatitis and MCC950 to specifically inhibit NLRP3; plasma was collected to measure cytokines and a marker of liver failure (alanine aminotransferase); liver tissues were collected and analyzed by quantitative polymerase chain reaction, immunohistochemistry, and flow cytometry.
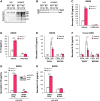
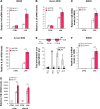
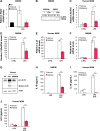
Results: In peritoneal macrophages, expression of NLRP3 and activation of its complex varied with time of day (circadian rhythm)-this regulation required NR1D1. Primary macrophages from Nr1d1-/- mice and human macrophages with knockdown of NR1D1 had altered expression patterns of NLRP3, compared to macrophages that expressed NR1D1, and altered patterns of IL1B and 1L18 production. Mice with disruption of Nr1d1 developed more-severe acute peritoneal inflammation and fulminant hepatitis than control mice. Incubation of macrophage with the NR1D1 activator SR9009 reduced expression of NLRP3 and secretion of cytokines. Mice given SR9009 developed less-severe liver failure and had longer survival times than mice given saline (control).
Conclusions: In studies of Nr1d1-/- mice and human macrophages with pharmacologic activation of NR1D1, we found NR1D1 to regulate the timing of NLRP3 expression and production of inflammatory cytokines by macrophages. Activation of NR1D1 reduced the severity of peritoneal inflammation and fulminant hepatitis in mice.
Keywords: Acute Liver Failure; Biological Clock; Immune Regulation; Rev-erbα.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors disclose no conflicts
Figures




References
-
- Cardoso FS, Marcelino P, Bagulho L, et al. Acute liver failure: An up-to-date approach. J Crit Care. 2017;39:25–30. - PubMed
-
- Bass J. Circadian topology of metabolism. Nature. 2012;491:348–356. - PubMed
-
- Curtis AM, Bellet MM, Sassone-Corsi P, et al. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. - PubMed
-
- Early JO, Curtis AM. Immunometabolism: Is it under the eye of the clock? Semin Immunol. 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

