Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate
- PMID: 29277680
- PMCID: PMC5880305
- DOI: 10.1016/j.jconrel.2017.12.023
Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate
Abstract
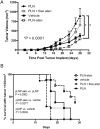
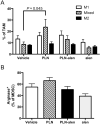
Liposomal nanoparticles are the most commonly used drug nano-delivery platforms. However, recent reports show that certain pegylated liposomal nanoparticles (PLNs) and polymeric nanoparticles have the potential to enhance tumor growth and inhibit antitumor immunity in murine cancer models. We sought herein to identify the mechanisms and determine whether PLN-associated immunosuppression and tumor growth can be reversed using alendronate, an immune modulatory drug. By conducting in vivo and ex vivo experiments with the immunocompetent TC-1 murine tumor model, we found that macrophages were the primary cells that internalized PLN in the tumor microenvironment and that PLN-induced tumor growth was dependent on macrophages. Treatment with PLN increased immunosuppression as evidenced by increased expression of arginase-1 in CD11b+Gr1+ cells, diminished M1 functionality in macrophages, and globally suppressed T-cell cytokine production. Encapsulating alendronate in PLN reversed these effects on myeloid cells and shifted the profile of multi-cytokine producing T-cells towards an IFNγ+ perforin+ response, suggesting increased cytotoxic functionality. Importantly, we also found that PLN-encapsulated alendronate (PLN-alen), but not free alendronate, abrogated PLN-induced tumor growth and increased progression-free survival. In summary, we have identified a novel mechanism of PLN-induced tumor growth through macrophage polarization and immunosuppression that can be targeted and inactivated to improve the anticancer efficacy of PLN-delivered drugs. Importantly, we also determined that PLN-alen not only reversed protumoral effects of the PLN carrier, but also had moderate antitumor activity. Our findings strongly support the inclusion of immune-responsive tumor models and in-depth immune functional studies in the preclinical drug development paradigm for cancer nanomedicines, and the further development of chemo-immunotherapy strategies to co-deliver alendronate and chemotherapy for the treatment of cancer.
Keywords: Alendronate; Cancer immunology; Immune modulation; Liposome; Nanoparticle.
Copyright © 2017. Published by Elsevier B.V.
Conflict of interest statement
All authors declare that they have no conflicts of interests.
Figures



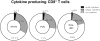

References
-
- Nakanishi W, Minami K, Shrestha LK, et al. Bioactive nanocarbon assemblies: nanoarchitectonics and applications. NanoToday. 2014;9:378–394.
-
- Hare JI, Lammers T, Ashford MB, et al. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. - PubMed
-
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. - PubMed
-
- Gabizon AA, Patil Y, La-Beck NM. New insights and evolving role of pegylated liposomal doxorubicin in cancer therapy. Drug Resist Updat. 2016;29:90–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

