The pathogenic roles of heparan sulfate deficiency in hereditary multiple exostoses
- PMID: 29277722
- PMCID: PMC6015767
- DOI: 10.1016/j.matbio.2017.12.011
The pathogenic roles of heparan sulfate deficiency in hereditary multiple exostoses
Abstract
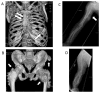
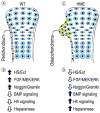
Heparan sulfate (HS) is an essential component of cell surface and matrix proteoglycans (HS-PGs) that include syndecans and perlecan. Because of their unique structural features, the HS chains are able to specifically interact with signaling proteins -including bone morphogenetic proteins (BMPs)- via their HS-binding domain, regulating protein availability, distribution and action on target cells. Hereditary Multiple Exostoses (HME) is a rare pediatric disorder linked to germline heterozygous loss-of-function mutations in EXT1 or EXT2 that encode Golgi-resident glycosyltransferases responsible for HS synthesis, resulting in a systemic HS deficiency. HME is characterized by cartilaginous/bony tumors -called osteochondromas or exostoses- that form within perichondrium in long bones, ribs and other elements. This review examines most recent studies in HME, framing them in the context of classic studies. New findings show that the spectrum of EXT mutations is larger than previously realized and the clinical complications of HME extend beyond the skeleton. Osteochondroma development requires a somatic "second hit" that would complement the germline EXT mutation to further decrease HS production and/levels at perichondrial sites of osteochondroma induction. Cellular studies have shown that the steep decreases in local HS levels: derange the normal homeostatic signaling pathways keeping perichondrium mesenchymal; cause excessive BMP signaling; and provoke ectopic chondrogenesis and osteochondroma formation. Data from HME mouse models have revealed that systemic treatment with a BMP signaling antagonist markedly reduces osteochondroma formation. In sum, recent studies have provided major new insights into the molecular and cellular pathogenesis of HME and the roles played by HS deficiency. These new insights have led to the first ever proof-of-principle demonstration that osteochondroma formation is a druggable process, paving the way toward the creation of a clinically-relevant treatment.
Keywords: Drug treatment; EXT1; EXT2; Heparan sulfate; Heparan sulfate proteoglycans; Hereditary multiple Exostoses; Signaling proteins and pathways.
Copyright © 2018. Published by Elsevier B.V.
Figures
References
-
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. nature. 2007;446:1030–1037. - PubMed
-
- Lamanna WC, Kalus I, Pavda M, Baldwin RJ, Merry CLR, Dierks T. The heparanome - The enigma of encoding and decoding heparan sulfate sulfation. J Biotechnology. 2007;129:290–307. - PubMed
-
- Dhoot GK, Gustafsson MK, Ai X, Sun w, Standiford DM, Emerson CP. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

