TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas
- PMID: 29278364
- PMCID: PMC5874661
- DOI: 10.3390/biomedicines6010004
TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas
Abstract
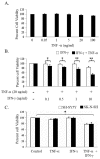
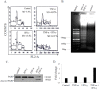
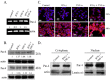
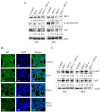
The objective of this study was to examine the combined effect of Interferon-gamma (IFN-γ) and Tumor Necrosis factor-alpha (TNF-α) on cytotoxicity and expression of prostate apoptosis response-4 (Par-4) and Par-4 interacting proteins B-cell lymphoma (Bcl-2), nuclear factor kappa-light-chain-enhancer of activated B cells/p65 subunit (NF-κB/p65), Ak mouse strain thymoma (Akt) in human neuroblastoma (NB) cells. Materials and methods included human neuroblastoma cell lines-SK-N-MC, SK-N-SH, and SH-SY5Y, which were treated with IFN-γ and TNF-α individually, or in combination, and were assessed for viability by tetrazolium (MTT) assay. Apoptosis was monitored by hypodiploid population (by flow cytometry), DNA fragmentation, Poly (ADP-ribose) polymerase (PARP) cleavage, and caspase-8 activity. Transcript level of Par-4 was measured by RT-PCR. Protein levels of Par-4 and suppressor of cytokine signaling 3 (SOCS-3) were assessed by immunoblotting. Cellular localization of Par-4 and p65 was examined by immunofluorescence. Unbiased transcript analysis for IFN-γ, TNF-α, and Par-4 were analyzed from three independent clinical datasets from neuroblastoma patients. In terms of results, SK-N-MC cells treated with a combination of, but not individually with, IFN-γ and TNF-α induced apoptosis characterized by hypodiploidy, DNA fragmentation, PARP cleavage, and increased caspase-8 activity. Apoptosis was associated with up-regulation of Par-4 mRNA and protein expression. Immunofluorescence studies revealed that Par-4 was localized exclusively in cytoplasm in SK-N-MC cells cultured for 24 h. but showed nuclear localization at 48 h. Treatment with IFN-γ and TNF-α together enhanced the intensity of nuclear Par-4. In gene expression, data from human neuroblastoma patients, levels of IFN-γ, and TNF-α have strong synergy with Par-4 expression and provide good survival advantage. The findings also demonstrated that apoptosis was associated with reduced level of pro-survival proteins-Bcl-2 and Akt and NF-κB/p65. Furthermore, the apoptotic effect induced by IFN-γ-induced Signal Transducer and Activator of Transcription-1(STAT-1), and could be due to down-regulation of suppressor of cytokine signaling-3 (SOCS3). The study concludes that a combinatorial approach using IFN-γ and TNF-α can be explored to maximize the effect in chemotherapy in neuroblastoma, and implies a role for Par-4 in the process.
Keywords: IFN-γ; NF-κB; Par-4; TNF-α; apoptosis; neuroblastoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
IFN-gamma prevents TNF-alpha-induced apoptosis in C2C12 myotubes through down-regulation of TNF-R2 and increased NF-kappaB activity.Cell Signal. 2005 Nov;17(11):1333-42. doi: 10.1016/j.cellsig.2005.02.001. Epub 2005 Mar 29. Cell Signal. 2005. PMID: 16125053
-
Synergistic induction of apoptosis in neuroblastoma cells using a combination of cytostatic drugs with interferon-gamma and TRAIL.Int J Oncol. 2004 Dec;25(6):1849-57. Int J Oncol. 2004. PMID: 15547726
-
Caspase-3 is involved in IFN-γ- and TNF-α-mediated MIN6 cells apoptosis via NF-κB/Bcl-2 pathway.Cell Biochem Biophys. 2013;67(3):1239-48. doi: 10.1007/s12013-013-9642-4. Cell Biochem Biophys. 2013. PMID: 23695786
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
Cited by
-
The Antitumor Potential of λ-Carrageenan Oligosaccharides on Gastric Carcinoma by Immunomodulation.Nutrients. 2023 Apr 24;15(9):2044. doi: 10.3390/nu15092044. Nutrients. 2023. PMID: 37432179 Free PMC article.
-
Prostate apoptosis response-4 and tumor suppression: it's not just about apoptosis anymore.Cell Death Dis. 2021 Jan 7;12(1):47. doi: 10.1038/s41419-020-03292-1. Cell Death Dis. 2021. PMID: 33414404 Free PMC article. Review.
-
Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy.Nat Commun. 2020 Jan 31;11(1):661. doi: 10.1038/s41467-020-14471-1. Nat Commun. 2020. PMID: 32005826 Free PMC article. Clinical Trial.
-
Inflammation and NF-κB Signaling in Prostate Cancer: Mechanisms and Clinical Implications.Cells. 2018 Aug 29;7(9):122. doi: 10.3390/cells7090122. Cells. 2018. PMID: 30158439 Free PMC article. Review.
-
Paeoniflorin Antagonizes TNF-α-Induced L929 Fibroblastoma Cells Apoptosis by Inhibiting NF-κBp65 Activation.Dose Response. 2018 Jun 4;16(2):1559325818774977. doi: 10.1177/1559325818774977. eCollection 2018 Apr-Jun. Dose Response. 2018. PMID: 29887769 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

