Bisphosphonates for advanced prostate cancer
- PMID: 29278410
- PMCID: PMC6486306
- DOI: 10.1002/14651858.CD006250.pub2
Bisphosphonates for advanced prostate cancer
Abstract
Background: The prevalence and incidence of pain and skeletal complications of metastatic bone disease such as pathologic fractures, spinal cord compression and hypercalcemia is high and an important contributor to morbidity, poor performance status and decreased quality of life. Moreover, pathologic fractures are associated with increased risk of death in people with disseminated malignancies. Therefore, prevention of pain and fractures are important goals in men with prostate cancer at risk for skeletal complications.
Objectives: To assess the effects of bisphosphonates in men with bone metastases from prostate cancer.
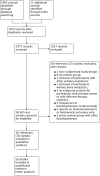
Search methods: We identified studies by electronic search of bibliographic databases including the Cochrane Controlled Trials Register and MEDLINE on 13 July 2017 and trial registries. We handsearched the Proceedings of American Society of Clinical Oncology (to July 2017) and reference lists of all eligible trials identified. This is an update of a review last published in 2006.
Selection criteria: We included randomized controlled studies comparing the effectiveness of bisphosphonates in men with bone metastases from prostate cancer.
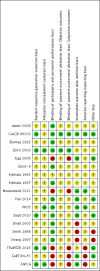
Data collection and analysis: Two review authors independently extracted data and assessed the quality of trials. We defined the proportion of participants with pain response as the primary end point; secondary outcomes were skeletal-related events, mortality, quality of life, adverse events, analgesic consumption and disease progression. We assessed the quality of the evidence for the main outcomes using the GRADE approach.
Main results: We included 18 trials reporting on 4843 participants comparing the effect of bisphosphonate administration to control regimens.
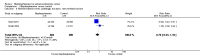
Primary outcome: there was no clear difference in the proportion of participants with pain response (RR 1.15, 95% CI 0.93 to 1.43; P = 0.20; I2 = 0%; 3 trials; 876 participants; low quality evidence). In absolute terms, bisphosphonates resulted in a pain response in 40 more participants per 1000 (19 fewer to 114 more).
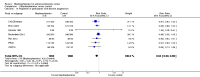
Secondary outcomes: bisphosphonates probably reduced the incidence of skeletal-related events in participants with prostate cancer metastatic to bone (RR 0.87, 95% CI 0.81 to 0.94; P = 0.27; I2 = 19%; 9 trials; 3153 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 58 fewer SREs per 1000 (85 fewer to 27 fewer).We found no clinically relevant differences in mortality (RR 0.97, 95% CI 0.91 to 1.04; P = 0.43; I2 = 1%; 9 trials; 2450 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 16 fewer deaths per 1000 (47 fewer to 21 more).Outcome definition of quality of life and the measurement tools varied greatly across trials and we were unable to extract any quantitative data for meta-analysis.Bisphosphonates probably increased the number of participants affected by nausea (RR 1.19, 95% CI 1.00 to 1.41; P = 0.05; I2 = 0%; 9 trials; 3008 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in seven more cases of nausea per 1000 (0 fewer to 14 more). Bisphosphonates probably increased the number of renal adverse events (RR 1.65, 95% CI 1.11 to 2.46; P = 0.01; I2 = 0%; 7 trials; 1794 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 22 more renal adverse events per 1000 (4 more to 50 more). We found no clear difference in the number of participants with osteonecrosis of the jaw between groups (RR 1.92, 95% CI 0.75 to 4.90; P = 0.17; I2 = 0%; 5 trials; 1626 participants; very low quality evidence). In absolute terms, bisphosphonates resulted in seven more cases with osteonecrosis of the jaw per 1000 (2 fewer to 29 more). We observed no clinically relevant difference in the proportion of participants with decreased analgesic consumption (RR 1.19, 95% CI 0.87 to 1.63; P = 0.28; I2 = 37%; 4 trials; 416 participants). Statistical analysis revealed that bisphosphonates probably reduced the number of participants with disease progression (RR 0.94, 95% CI 0.90 to 0.98; P = 0.006; I2 = 0%; 7 trials; 2115 participants; moderate quality evidence). In absolute terms, bisphosphonates resulted in 36 fewer cases of disease progression per 1000 (71 fewer to 7 fewer).Findings of our predefined subgroup and sensitivity analyses were no different from those of the primary analyses.
Authors' conclusions: Based on low quality evidence, there may be no clinically relevant difference in the proportion of men with pain response between bisphosphonates and control regimens in men with bone metastases from prostate cancer. Bisphosphonates probably decrease the number of skeletal-related events and disease progression. These benefits need to be weighed against the increased risk of renal impairment and nausea in men receiving bisphosphonates. Future studies should explicitly evaluate patient important outcomes such as quality of life and pain by using standardized and comparable assessment tools.
Conflict of interest statement
SM: none known.
IM: none known.
FJ: received payment for lectures from MSD, Riemser and Tesaro; received travel, accommodation or meeting expenses from Pfizer, Roche, Tesaro.
KJ: received payment for lectures from Amgen.
KKY: none known.
AH: none known.
NS: none known.
Figures
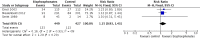


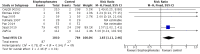






























Update of
-
Bisphosphonates for advanced prostate cancer.Cochrane Database Syst Rev. 2006 Oct 18;(4):CD006250. doi: 10.1002/14651858.CD006250. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Dec 26;12:CD006250. doi: 10.1002/14651858.CD006250.pub2. PMID: 17054286 Updated.
References
References to studies included in this review
Abetz 2006 {published data only}
-
- Abetz L, Barghout V, Arbuckle R, Bosch V, Shirina N, Saad F. Impact of zoledronic acid (Z) on pain in prostate cancer patients with bone metastases in a randomised placebo‐control trial. Journal of Clinical Oncology 2006:4638.
-
- Barghout V, Abetz L, Arbuckle R, Bosch V, Hei Y, Saad F. Effect of zoledronic acid (Z) on pain in prostate cancer patients with bone metastases based on performance status. Journal of Clinical Oncology ‐ Supplement 2006;24(18):14544.
CALGB 90202 {published data only}
Elomaa 1992 {published data only}
-
- Elomaa I, Kylmata T, Tammela T, Vitanen J, Ottelin M, Ruutu K, et al. Effect of oral clodronate on bone pain: a controlled study in patients with metastatic prostate cancer. International Journal of Urology and Nephrology 1992;24(2):159‐66. - PubMed
Ernst 2003 {published data only}
-
- Ernst DS, Tannock IF, Winquist EW, Venner PM, Reyno L, Moore MJ, et al. Randomized, double‐blind, controlled trial of mitoxantron/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone‐refractory prostate cancer and pain. Journal of Clinical Oncology 2003;21(17):3335‐42. - PubMed
Figg 2005 {published data only}
-
- Figg WD, Liu Y, Arlen P, Gulley J, Steinberg SM, Liewehr DJ, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. Journal of Urology 2005;173:790‐6. - PubMed
GU02‐4 {published data only}
-
- Hahn NM, Yiannoutsos CT, Kirkpatrick K, Sharma J, Sweeney CJ. Failure to suppress markers of bone turnover on first‐line hormone therapy for metastatic prostate cancer is associated with shorter time to skeletal‐related event. Clinical Genitourinary Cancer 2014;12(1):33‐40.e4. [PUBMED: 24126237] - PubMed
-
- Sharma J, Yiannoutsos CT, Hahn NM, Sweeney C. Prognostic value of suppressed markers of bone turnover (BTO) after 6 months of androgen deprivation therapy (ADT) in prostate cancer. Journal of Clinical Oncology 2011;29:4594.
-
- Sweeney C, Dugan WM, Dreicer R, Chu F, Parks G, Baker K, et al. A randomized placebo‐controlled trial of daily high‐dose oral risedronate in men with metastatic prostate cancer commencing androgen deprivation therapy (ADT). Journal of Clinical Oncology 2010;28:e15000.
Kylmala 1993 {published data only}
-
- Kylmala T, Tammela T, Risteli L, Risteli J, Taube T, Elomma I. Evaluation of the effect of oral clodronate on skeletal metastases with type I collagen metabolites. A controlled trial of the Finnish Prostate Cancer Group. European Journal of Cancer 1993;29A(6):821‐5. - PubMed
Kylmala 1997 {published data only}
Meulenbeld 2012 {published data only}
-
- Meulenbeld HJ, Werkhoven ED, Coenen JLLM, Creemers GJ, Loosveld OJL, Jong PC, et al. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic Castration Resistant Prostate Cancer (CRPC), the Netherlands Prostate Study (NePro). European Journal of Cancer 2012;48:2993‐3000. - PubMed
Pan 2014 {published data only}
-
- Pan Y, Jin H, Chen W, Yu Z, Ye T, Zheng Y, et al. Docetaxel with or without zoledronic acid for castration‐resistant prostate cancer. International Urology and Nephrology 2014;46:2319‐26. - PubMed
PR05 {published data only}
-
- Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson ACR, et al. A double‐blind, placebo‐controlled, randomised trial of oral sodium clodronate for metastatic prostate cancer (MRC PRO5 Trial). Journal of the National Cancer Institute 2003;95(17):1300‐11. - PubMed
Saad 2010 {published data only}
-
- Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology 2010;76:1175‐81. - PubMed
-
- Saad F, Gleason DM, Murray R. A randomized, placebo‐controlled trial of zoledronic acid in patients with hormone‐refractory metastatic prostate carcinoma. Journal of the National Cancer Institute 2002;94(19):1458‐68. - PubMed
-
- Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long‐term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone‐refractory prostate cancer. Journal of the National Cancer Institute 2004;96:879‐82. - PubMed
-
- Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F. Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Annals of Oncology 2006;17:986‐9. - PubMed
Small 2003 {published data only}
-
- Small EJ, Matthew RS, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo‐controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostatic cancer. Journal of Clinical Oncology 2003;21(23):4277‐84. - PubMed
Smith 1989 {published data only}
-
- Smith JA Jr. Palliation of painful bone metastases from prostate cancer using sodium etidronate: results of a randomized, prospective, double‐blind, placebo‐controlled study. Journal of Urology 1989;141:85‐7. - PubMed
Strang 1997 {published data only}
-
- Strang P, Nilsson S, Brandstedt S. The analgesic efficacy of clodronate compared with placebo inpatients with painful bone metastases from prostatic cancer. Anticancer Research 1997;17:4717‐21. - PubMed
TRAPEZE 2016 {published data only}
-
- James N, Pirrie S, Pope A, Barton D, Andronis L, Goranitis I, et al. TRAPEZE: A randomised controlled trial of the clinical effectiveness and cost‐effectiveness of chemotherapy with zoledronic acid, strontium‐89, or both, in men with bony metastatic castration‐refractory prostate cancer. Health Technology Assessment 2016;20(53):1‐127. - PMC - PubMed
-
- James ND, Andronis L, Goranitis I, Pirrie S, Pope A, Barton D, et al. Cost‐effectiveness of zoledronic acid and strontium‐89 as bone protecting treatments in addition to chemotherapy in patients with metastatic castrate‐refractory prostate cancer. (ISRCTN 12808747) TRAPEZE. Journal of Clinical Oncology 2015;33:e16108. - PubMed
-
- James ND, Pirrie S, Barton D, Brown JE, Billingham L, Collins SI, et al. Clinical outcomes in patients with castrate‐refractory prostate cancer (CRPC) metastatic to bone randomized in the factorial TRAPEZE trial to docetaxel (D) with strontium‐89 (Sr89), zoledronic acid (ZA), neither, or both (ISRCTN 12808747). Journal of Clinical Oncology 2013;31:LBA5000.
-
- James ND, Pirrie SJ, Pope AM, Barton D, Andronis L, Goranitis I, et al. Clinical outcomes and survival following treatment of metastatic castrate‐refractory prostate cancer with docetaxel alone or with strontium‐89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncology 2016;2(4):493‐9. [PUBMED: 26794729] - PubMed
-
- Porfiri E, Collins SI, Barton D, Billingham L, McLaren D, Nixon GG, et al. Initial feasibility and safety results from a phase II/III clinical trial to evaluate docetaxel (D) therapy in combination with zoledronic acid (ZA) {+/‐} strontium‐89 (Sr89) in hormone‐refractory prostate cancer patients: ISRCTN12808747. Journal of Clinical Oncology 2010;28:4677.
ZABTON‐PC {published data only}
-
- Ueno S, Mizokami A, Fukagai T, Fujimoto N, Oh‐Oka H, Kondo Y, et al. Efficacy of combined androgen blockade with zoledronic acid treatment in prostate cancer with bone metastasis: the ZABTON‐PC (zoledronic acid/androgen blockade trial on prostate cancer) study. Anticancer Research 2013;33:3837‐44. - PubMed
ZAPCA {published data only}
-
- Kamba T, Kamoto T, Maruo S, Kikuchi T, Shimizu Y, Namiki S, et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. International Journal of Clinical Oncology 2017;22:166‐73. - PubMed
-
- Kamba T, Kamoto T, Shimizu Y, Namiki S, Fujimoto K, Kawanishi H, et al. A phase III, multicenter, randomized, controlled study of maximum androgen blockade with versus without zoledronic acid in treatment‐naive prostate cancer patients with bone metastases: Results of ZAPCA study. Journal of Clinical Oncology 2015;33:150. - PubMed
References to studies excluded from this review
Adami 1985 {published data only}
-
- Adami S, Salvagno G, Guarrera G. Dichloromethylene‐diphosphonate in patients with prostatic carcinoma metastatic to the skeleton. Journal of Urology 1985;134:1152‐4. - PubMed
Adami 1989 {published data only}
-
- Adami S, Mian M. Clodronate therapy of metastatic bone disease in patients with prostatic carcinoma. Recent Results in Cancer Research 1989;116:67‐72. - PubMed
BO18039 {published data only (unpublished sought but not used)}
-
- BO18039. Randomized, two arm, placebo controlled double dummy study to compare the efficacy of intravenous loading doses followed by maintenance treatment with oral ibandronic acid versus zoledronic acid in patients with malignant and painful bone disease. www.clinicaltrialsregister.eu/ctr‐search/search?query=2004‐001854‐10 Date first received: 16 February 2005.
CALGB 70604 {published data only}
-
- Himelstein AL, Qin R, Novotny PJ, Seisler DK, Khatcheressian JL, Roberts JD, et al. CALGB 70604 (Alliance): a randomized phase III study of standard dosing vs. longer interval dosing of zoledronic acid in metastatic cancer. Journal of Clinical Oncology 2015;33:9501.
Carey 1988 {published data only}
-
- Carey PO, Lippert MC. Treatment of painful prostatic bone metastases with oral etidronate disodium. Urology 1988;32:403‐7. - PubMed
Clarke 1991 {published data only}
Cresswell 1995 {published data only}
-
- Cresswell SM, English PJ, Hall RR. Pain relief and quality‐of‐life assessment following intravenous and oral clodronate in hormone‐escaped metastatic prostate cancer. British Journal of Urology 1995;76:360‐5. - PubMed
Fernandez‐Conde 1997 {published data only}
-
- Fernandez‐Conde M, Alcover J, Aaron JE, Ordi J, Carretero P. Skeletal response to clodronate in prostate cancer with bone metastases. American Journal of Clinical Oncology 1997;20(5):471‐6. - PubMed
Fizazi 2009 {published data only}
-
- Fizazi K, Bosserman L, Gao G, Skacel T, Markus R. Denosumab treatment of prostate cancer with bone metastases and increased urine N‐telopeptide levels after therapy with intravenous bisphosphonates: results of a randomized phase II trial. Journal of Urology 2009;182:509‐15; discussion 515‐6. - PubMed
Fizazi 2011 {published data only}
-
- Fizazi K, Carducci MA, Smith MR, Damiao R, Brown JE, Karsh L, et al. A randomized phase III trial of denosumab versus zoledronic acid in patients with bone metastases from castration‐resistant prostate cancer [abstract no. LBA4507]. Journal of Clinical Oncology 2010;28(18):951.
-
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. Journal of Clinical Oncology 2009;27:1564‐71. - PubMed
Heidenreich 2001 {published data only}
-
- Heidenreich A, Hoffmann R, Engelmann UH. The use of bisphosphonate for the palliative treatment of painful bone metastasis due to hormone refractory prostate cancer. Journal of Urology 2001;165(1):136‐40. - PubMed
Heidenreich 2002 {published data only}
-
- Heidenreich A, Elert A, Hofmann R. Ibandronate in the treatment of prostate cancer associated painful osseous metastases. Prostate Cancer and Prostatic Diseases 2002;5(3):231‐5. - PubMed
Jagdev 2001 {published data only}
-
- Jagdev SP, Purohit OP, Heatley S, Herling C, Coleman RE. Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Annals of Oncology 2001;12:1433‐8. - PubMed
Kylmala 1994 {published data only}
-
- Kylmala T, Tammela TL, Lindholm TS. The effect of combined intravenous and oral clodronate treatment on bone pain in patients with metastatic prostate cancer. Annales Chirurgiae et Gynaecologiae 1994;83(4):316‐9. - PubMed
Magnusson 1998 {published data only}
-
- Magnusson P, Larssson L, Englund G, Larsson B, Strang P, Selin‐Sjogren L. Differences of bone alkaline phosphatase isoforms in metastatic bone disease and discrepant effects of clodronate on different skeletal sites indicated by location of pain. Clinical Chemistry 1998;44(8 Pt 1):1621‐8. - PubMed
MER‐101‐03 {published data only}
-
- McHugh C, Madigan K, Walsh A, Fox J, Leonard TW, Quint JB. MER‐101‐03, a multicenter, phase II study to compare MER‐101 20mg tablets to intravenous zoledronic acid 4mg in prostate cancer patients. Journal of Clinical Oncology 2009;27:5161.
Pelger 1998 {published data only}
-
- Pelger RC, Hamdy NA, Zwinderman AH. Effects of the bisphosphonate olpadronate in patients with carcinoma of prostate metastatic to the skeleton. Bone 1998;22:403‐8. - PubMed
STAMPEDE {published data only}
-
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387(10024):1163‐77. [PUBMED: 26719232] - PMC - PubMed
-
- James ND, Sydes MR, Mason MD, Clarke NW, Dearnaley DP, Spears MR, et al. Docetaxel and/or zoledronic acid for hormone‐naive prostate cancer: first overall survival results from STAMPEDE (NCT00268476). Journal of Clinical Oncology 2015;33:5001.
Taube 1994 {published data only}
-
- Taube T, Kylmala T, Lamberg‐Allardt C. The effect of clodronate on bone in metastatic prostate cancer: histomorphonmetric report of a double‐blind randomised placebo‐controlled study. European Journal of Cancer 1994;30:751‐8. - PubMed
Vorreuther 1992 {published data only}
-
- Vorreuther R, Klotz T, Engelking R. Clodronate in the palliative therapy of bone‐metastasized prostate carcinoma. Urologe A 1992;31(2):63‐6. - PubMed
Vorreuther 1993 {published data only}
-
- Vorreuther R. Bisphosphonates as an adjunct to palliative therapy of bone metastases from prostate carcinoma. A pilot study on clodronate. British Journal of Urology 1993;72((5 Pt 2)):792‐5. - PubMed
Wang 2013 {published data only}
-
- Wang F, Chen W, Chen H, Mo L, Jin H, Yu Z, et al. Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Medical Oncology 2013;30:657. - PubMed
Additional references
Alibhai 2017
-
- Alibhai SMH, Zukotynski K, Walker‐Dilks C, Emmenegger U, Finelli A, Morgan SC, et al. Bone Health and Bone‐targeted Therapies for Prostate Cancer: a Programme in Evidence‐based Care ‐ Cancer Care Ontario Clinical Practice Guideline. Clinical oncology (Royal College of Radiologists (Great Britain)) 2017;29(6):348‐55. [PUBMED: 28169118] - PubMed
Bartl 2007
-
- Bartl R, Frisch B, Tresckow E, Bartl C. Bisphosphonates in Medical Practice ‐ Actions, Side effects, Indications, Strategies. Berlin (Germany): Springer, 2007.
Bartl 2008
-
- Bartl R, Bartl C, Gradinger R. Use of bisphosphonates in orthopedic surgery [Einsatz der Bisphosphonate in der Orthopädie und Unfallchirurgie]. Der Orthopäde 2008;37(6):595‐614. - PubMed
Bubendorf 2000
-
- Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathology 2000;31(5):578‐83. - PubMed
Clohisy 2002
-
- Clohisy DR, Mantyh PW. Bone cancer pain. Cancer 2002;97(3 Suppl):866‐73. - PubMed
Coleman 1997
-
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80(8 Suppl):1588‐94. - PubMed
Coleman 2012
-
- Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone‐targeted agents on cancer progression and mortality. Journal of the National Cancer Institute 2012;104(14):1059‐67. - PubMed
Conford 2017
-
- Cornford P, Bellmunt J, Bolla M, Briers E, Santis M, Gross T. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration‐resistant prostate cancer. European Urology 2017;71(4):631‐42. - PubMed
Cookson 2013
-
- Cookson MS, Roth BJ, Dahm P, Engstrom C, Freedland SJ, Hussain M. Castration‐resistant prostate cancer: AUA guideline. Journal of Urology 2013;190(2):429‐38. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Devogelaer 2000
-
- Devogelaer D. Treatment of bone diseases with bisphosphonates, excluding osteoporosis. Current Opinion in Rheumatology 2000;12:331‐5. - PubMed
El‐Amm 2016
Ferlay 2013
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 11, 2013. globocan.iarc.fr (accessed 12 August 2017).
Fitzpatrick 2014
-
- Fitzpatrick JM, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, Tombal B, et al. Optimal management of metastatic castration‐resistant prostate cancer: highlights from a European Expert Consensus Panel. European Journal of Cancer 2014;50(9):1617‐27. - PubMed
Fizazi 2015
-
- Fizazi K, Massard C, Smith M, Rader M, Brown J, Milecki P, et al. Bone‐related parameters are the main prognostic factors for overall survival in men with bone metastases from castration‐resistant prostate cancer. European Urology 2015;68(1):42‐50. - PubMed
Gartrell 2014
Gartrell 2015
-
- Gartrell BA, Coleman R, Efstathiou E, Fizazi K, Logothetis CJ, Smith MR, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. European Urology 2015;68(5):850‐8. - PubMed
Hellstein 2011
-
- Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. Journal of the American Dental Association 2011;142(11):1243‐51. - PubMed
Higgins 2011a
-
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horwich 2013
-
- Horwich A, Parker C, Reijke T, Kataja V. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2013;24 Suppl 6:vi106‐14. - PubMed
Howlader 2015
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER cancer statistics review, 1975‐2012, based on November 2014 SEER data submission, posted to the SEER website, April 2015. seer.cancer.gov/csr/1975_2012/ (accessed prior to 26 November 2017).
Lee 2014
-
- Lee SH, Chan RC, Chang SS, Tan YL, Chang KH, Lee MC, et al. Use of bisphosphonates and the risk of osteonecrosis among cancer patients: a systemic review and meta‐analysis of the observational studies. Supportive Care in Cancer 2014;22(2):553‐60. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liu 2015
Mhaskar 2012
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. - PubMed
Mohler 2016
-
- Mohler JL, Armstrong AJ, Bahnson RR, D'Amico AV, Davis BJ, Eastham JA, et al. Prostate cancer, version 1.2016. Journal of the National Comprehensive Cancer Network 2016;14(1):19‐30. - PubMed
Newcomb 2010
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Reid 2003
-
- Reid IR. Bisphosphonates: new indications and methods of administration. Current Opinion in Rheumatology 2003;15:458‐63. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2016
-
- Reyes C, Hitz M, Prieto‐Alhambra D, Abrahamsen B. Risks and benefits of bisphosphonate therapies. Journal of Cellular Biochemistry 2016;117(1):20‐8. - PubMed
Saunders 2004
-
- Saunders Y, Ross JR, Broadley KE, Edmonds PM, Patel S. Systematic review of bisphosphonates for hypercalcaemia of malignancy. Palliative Medicine 2004;18(5):418‐31. - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thosani 2013
-
- Thosani N, Thosani SN, Kumar S, Nugent Z, Jimenez C, Singh H, et al. Reduced risk of colorectal cancer with use of oral bisphosphonates: a systematic review and meta‐analysis. Journal of Clinical Oncology 2013;31(5):623‐30. - PubMed
Tierney 2007
Vignani 2016
-
- Vignani F, Bertaglia V, Buttigliero C, Tucci M, Scagliotti GV, Maio M. Skeletal metastases and impact of anticancer and bone‐targeted agents in patients with castration‐resistant prostate cancer . Cancer Treatment Reviews 2016;44:61‐73. - PubMed
Wirth 2016
-
- Wirth M, Berges R, Fröhner M, Miller K, Rübben H, Stöckle M, et al. Interdisziplinäre Leitlinie der Qualität S3 zur Früherkennung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Langversion 4.0. Berlin (Germany): AWMF, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

