Inositol Polyphosphate Multikinase Inhibits Angiogenesis via Inositol Pentakisphosphate-Induced HIF-1α Degradation
- PMID: 29279301
- PMCID: PMC5805644
- DOI: 10.1161/CIRCRESAHA.117.311983
Inositol Polyphosphate Multikinase Inhibits Angiogenesis via Inositol Pentakisphosphate-Induced HIF-1α Degradation
Abstract
Rationale: Inositol polyphosphate multikinase (IPMK) and its major product inositol pentakisphosphate (IP5) regulate a variety of cellular functions, but their role in vascular biology remains unexplored.
Objective: We have investigated the role of IPMK in regulating angiogenesis.
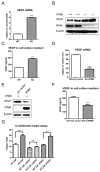
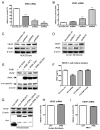
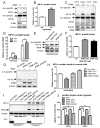
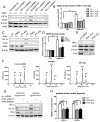
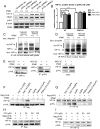
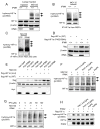
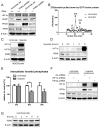
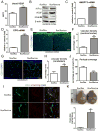
Methods and results: Deletion of IPMK in fibroblasts induces angiogenesis in both in vitro and in vivo models. IPMK deletion elicits a substantial increase of VEGF (vascular endothelial growth factor), which mediates the regulation of angiogenesis by IPMK. The regulation of VEGF by IPMK requires its catalytic activity. IPMK is predominantly nuclear and regulates gene transcription. However, IPMK does not apparently serve as a transcription factor for VEGF. HIF (hypoxia-inducible factor)-1α is a major determinant of angiogenesis and induces VEGF transcription. IPMK deletion elicits a major enrichment of HIF-1α protein and thus VEGF. HIF-1α is constitutively ubiquitinated by pVHL (von Hippel-Lindau protein) followed by proteasomal degradation under normal conditions. However, HIF-1α is not recognized and ubiquitinated by pVHL in IPMK KO (knockout) cells. IP5 reinstates the interaction of HIF-1α and pVHL. HIF-1α prolyl hydroxylation, which is prerequisite for pVHL recognition, is interrupted in IPMK-deleted cells. IP5 promotes HIF-1α prolyl hydroxylation and thus pVHL-dependent degradation of HIF-1α. Deletion of IPMK in mouse brain increases HIF-1α/VEGF levels and vascularization. The increased VEGF in IPMK KO disrupts blood-brain barrier and enhances brain blood vessel permeability.
Conclusions: IPMK, via its product IP5, negatively regulates angiogenesis by inhibiting VEGF expression. IP5 acts by enhancing HIF-1α hydroxylation and thus pVHL-dependent degradation of HIF-1α.
Keywords: Egln1 protein; blood–brain barrier; hydroxylation; inositol phosphates; vascular endothelial growth factor A; von Hippel-Lindau protein.
© 2017 American Heart Association, Inc.
Figures
Similar articles
-
Runx2 protein stabilizes hypoxia-inducible factor-1α through competition with von Hippel-Lindau protein (pVHL) and stimulates angiogenesis in growth plate hypertrophic chondrocytes.J Biol Chem. 2012 Apr 27;287(18):14760-71. doi: 10.1074/jbc.M112.340232. Epub 2012 Feb 20. J Biol Chem. 2012. PMID: 22351759 Free PMC article.
-
Wogonin inhibits tumor angiogenesis via degradation of HIF-1α protein.Toxicol Appl Pharmacol. 2013 Sep 1;271(2):144-55. doi: 10.1016/j.taap.2013.04.031. Epub 2013 May 23. Toxicol Appl Pharmacol. 2013. PMID: 23707765
-
Topotecan blocks hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression induced by insulin-like growth factor-I in neuroblastoma cells.Cancer Res. 2005 Jun 1;65(11):4775-81. doi: 10.1158/0008-5472.CAN-04-3332. Cancer Res. 2005. PMID: 15930297
-
Critical role of hypoxia sensor--HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing.Curr Med Chem. 2012;19(1):90-7. doi: 10.2174/092986712803413944. Curr Med Chem. 2012. PMID: 22300081 Review.
-
The Expanding Significance of Inositol Polyphosphate Multikinase as a Signaling Hub.Mol Cells. 2017 May 31;40(5):315-321. doi: 10.14348/molcells.2017.0066. Epub 2017 May 29. Mol Cells. 2017. PMID: 28554203 Free PMC article. Review.
Cited by
-
HIF-1α Promotes the Metastasis of Esophageal Squamous Cell Carcinoma by Targeting SP1.J Cancer. 2020 Jan 1;11(1):229-240. doi: 10.7150/jca.35537. eCollection 2020. J Cancer. 2020. PMID: 31892989 Free PMC article.
-
The Inositol Phosphate System-A Coordinator of Metabolic Adaptability.Int J Mol Sci. 2022 Jun 16;23(12):6747. doi: 10.3390/ijms23126747. Int J Mol Sci. 2022. PMID: 35743190 Free PMC article. Review.
-
Functions, Mechanisms, and therapeutic applications of the inositol pyrophosphates 5PP-InsP5 and InsP8 in mammalian cells.J Cardiovasc Transl Res. 2024 Feb;17(1):197-215. doi: 10.1007/s12265-023-10427-0. Epub 2023 Aug 24. J Cardiovasc Transl Res. 2024. PMID: 37615888 Review.
-
Kindlin-3 Promotes Angiogenesis via Notch Signalling and Is Crucial for Functional Recovery Postmyocardial Infarction.J Cell Mol Med. 2025 Mar;29(6):e70494. doi: 10.1111/jcmm.70494. J Cell Mol Med. 2025. PMID: 40099947 Free PMC article.
-
Inositol polyphosphates promote T cell-independent humoral immunity via the regulation of Bruton's tyrosine kinase.Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12952-12957. doi: 10.1073/pnas.1821552116. Epub 2019 Jun 12. Proc Natl Acad Sci U S A. 2019. PMID: 31189594 Free PMC article.
References
-
- Monserrate JP, York JD. Inositol phosphate synthesis and the nuclear processes they affect. Curr Opin Cell Biol. 2010;22(3):365–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

