Structure-function insights into direct lipid transfer between membranes by Mmm1-Mdm12 of ERMES
- PMID: 29279306
- PMCID: PMC5839780
- DOI: 10.1083/jcb.201704119
Structure-function insights into direct lipid transfer between membranes by Mmm1-Mdm12 of ERMES
Abstract
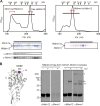
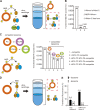
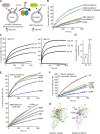
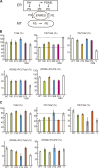
The endoplasmic reticulum (ER)-mitochondrial encounter structure (ERMES) physically links the membranes of the ER and mitochondria in yeast. Although the ER and mitochondria cooperate to synthesize glycerophospholipids, whether ERMES directly facilitates the lipid exchange between the two organelles remains controversial. Here, we compared the x-ray structures of an ERMES subunit Mdm12 from Kluyveromyces lactis with that of Mdm12 from Saccharomyces cerevisiae and found that both Mdm12 proteins possess a hydrophobic pocket for phospholipid binding. However in vitro lipid transfer assays showed that Mdm12 alone or an Mmm1 (another ERMES subunit) fusion protein exhibited only a weak lipid transfer activity between liposomes. In contrast, Mdm12 in a complex with Mmm1 mediated efficient lipid transfer between liposomes. Mutations in Mmm1 or Mdm12 impaired the lipid transfer activities of the Mdm12-Mmm1 complex and furthermore caused defective phosphatidylserine transport from the ER to mitochondrial membranes via ERMES in vitro. Therefore, the Mmm1-Mdm12 complex functions as a minimal unit that mediates lipid transfer between membranes.
© 2018 Kawano et al.
Figures


References
-
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.-W., Kapral G.J., Grosse-Kunstleve R.W., et al. . 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
-
- Berendsen H.J.C., Postma J.P.M., van Gunsteren W.F., DiNola A., and Haak J.R.. 1984. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81:3684–3690. 10.1063/1.448118 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

