Angiogenic factor imbalance precedes complement deposition in placentae of the BPH/5 model of preeclampsia
- PMID: 29279353
- PMCID: PMC5901389
- DOI: 10.1096/fj.201701008R
Angiogenic factor imbalance precedes complement deposition in placentae of the BPH/5 model of preeclampsia
Abstract
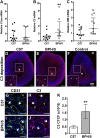
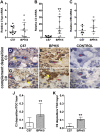
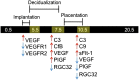
Preeclampsia (PE), a hypertensive disorder of pregnancy, is a leading cause of maternal and fetal morbidity and mortality. Although the etiology is unknown, PE is thought to be caused by defective implantation and decidualization in pregnancy. Pregnant blood pressure high (BPH)/5 mice spontaneously develop placentopathies and maternal features of human PE. We hypothesized that BPH/5 implantation sites have transcriptomic alterations. Next-generation RNA sequencing of implantation sites at peak decidualization, embryonic day (E)7.5, revealed complement gene up-regulation in BPH/5 vs. controls. In BPH/5, expression of complement factor 3 was increased around the decidual vasculature of E7.5 implantation sites and in the trophoblast giant cell layer of E10.5 placentae. Altered expression of VEGF pathway genes in E5.5 BPH/5 implantation sites preceded complement dysregulation, which correlated with abnormal vasculature and increased placental growth factor mRNA and VEGF164 expression at E7.5. By E10.5, proangiogenic genes were down-regulated, whereas antiangiogenic sFlt-1 was up-regulated in BPH/5 placentae. We found that early local misexpression of VEGF genes and abnormal decidual vasculature preceded sFlt-1 overexpression and increased complement deposition in BPH/5 placentae. Our findings suggest that abnormal decidual angiogenesis precedes complement activation, which in turn contributes to the aberrant trophoblast invasion and poor placentation that underlie PE.-Sones, J. L., Merriam, A. A., Seffens, A., Brown-Grant, D.-A., Butler, S. D., Zhao, A. M., Xu, X., Shawber, C. J., Grenier, J. K., Douglas, N. C. Angiogenic factor imbalance precedes complement deposition in placentae of the BPH/5 model of preeclampsia.
Keywords: RGC32; VEGF; decidual vasculature; implantation; sFlt-1; trophoblast.
Conflict of interest statement
The authors thank Robin L. Davisson for the generous gift of BPH/5 mice and additional support; Michelle A. Hoch for assistance with data analysis; Jan K. Kitajewski and Virginia E. Papaioannou for in-depth discussions and assistance with manuscript editing; and Theresa Swayne and Emilia Laura Munteanu for technical assistance with confocal microscopy. This work was supported by U.S. National Institutes of Health (NIH) National Heart Lung and Blood Institute Grant 1R01HL127013-01A1 (to N.C.D) and NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P50-HD076210 (to J.L.S. and J.K.G.). Immunofluorescence images were collected in the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University (New York, NY, USA), supported by NIH National Cancer Institute Grant P30 CA013696. The confocal microscope was purchased with NIH National Center for Research Resources Grant S10 RR025686. The authors declare no conflicts of interest.
Figures





References
-
- American College of Obstetricians and Gynecologists ; Task Force on Hypertension in Pregnancy . (2013) Hypertension in pregnancy: report of the American College of Obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol. 122, 1122–1131 10.1097/01.AOG.0000437382.03963.88 - DOI - PubMed
-
- Tranquilli A. L., Dekker G., Magee L., Roberts J., Sibai B. M., Steyn W., Zeeman G. G., Brown M. A. (2014) The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 4, 97–104 10.1016/j.preghy.2014.02.001 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

