Adaptive and Reversible Resistance to Kras Inhibition in Pancreatic Cancer Cells
- PMID: 29279356
- PMCID: PMC5837062
- DOI: 10.1158/0008-5472.CAN-17-2129
Adaptive and Reversible Resistance to Kras Inhibition in Pancreatic Cancer Cells
Abstract
Activating mutations in KRAS are the hallmark genetic alterations in pancreatic ductal adenocarcinoma (PDAC) and the key drivers of its initiation and progression. Longstanding efforts to develop novel KRAS inhibitors have been based on the assumption that PDAC cells are addicted to activated KRAS, but this assumption remains controversial. In this study, we analyzed the requirement of endogenous Kras to maintain survival of murine PDAC cells, using an inducible shRNA-based system that enables temporal control of Kras expression. We found that the majority of murine PDAC cells analyzed tolerated acute and sustained Kras silencing by adapting to a reversible cell state characterized by differences in cell morphology, proliferative kinetics, and tumor-initiating capacity. While we observed no significant mutational or transcriptional changes in the Kras-inhibited state, global phosphoproteomic profiling revealed significant alterations in cell signaling, including increased phosphorylation of focal adhesion pathway components. Accordingly, Kras-inhibited cells displayed prominent focal adhesion plaque structures, enhanced adherence properties, and increased dependency on adhesion for viability in vitro Overall, our results call into question the degree to which PDAC cells are addicted to activated KRAS, by illustrating adaptive nongenetic and nontranscriptional mechanisms of resistance to Kras blockade. However, by identifying these mechanisms, our work also provides mechanistic directions to develop combination strategies that can help enforce the efficacy of KRAS inhibitors.Significance: These results call into question the degree to which pancreatic cancers are addicted to KRAS by illustrating adaptive nongenetic and nontranscriptional mechanisms of resistance to Kras blockade, with implications for the development of KRAS inhibitors for PDAC treatment. Cancer Res; 78(4); 985-1002. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



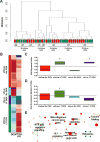
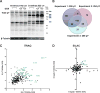
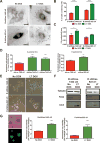
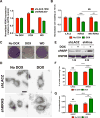
References
-
- Ryan DP, Hong TS, Bardeesy N. Pancreatic Adenocarcinoma. NEJM. 2014;371:1039–49. - PubMed
-
- American Cancer Society. Cancer Facts and Figures 2017. Atlanta: A; 2017.
-
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

