From in silico hit to long-acting late-stage preclinical candidate to combat HIV-1 infection
- PMID: 29279368
- PMCID: PMC5789948
- DOI: 10.1073/pnas.1717932115
From in silico hit to long-acting late-stage preclinical candidate to combat HIV-1 infection
Erratum in
-
Correction for Kudalkar et al., From in silico hit to long-acting late-stage preclinical candidate to combat HIV-1 infection.Proc Natl Acad Sci U S A. 2018 Mar 20;115(12):E2899. doi: 10.1073/pnas.1803199115. Epub 2018 Mar 12. Proc Natl Acad Sci U S A. 2018. PMID: 29531046 Free PMC article. No abstract available.
Abstract
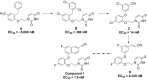
The HIV-1 pandemic affecting over 37 million people worldwide continues, with nearly one-half of the infected population on highly active antiretroviral therapy (HAART). Major therapeutic challenges remain because of the emergence of drug-resistant HIV-1 strains, limitations because of safety and toxicity with current HIV-1 drugs, and patient compliance for lifelong, daily treatment regimens. Nonnucleoside reverse transcriptase inhibitors (NNRTIs) that target the viral polymerase have been a key component of the current HIV-1 combination drug regimens; however, these issues hamper them. Thus, the development of novel more effective NNRTIs as anti-HIV-1 agents with fewer long-term liabilities, efficacy on new drug-resistant HIV-1 strains, and less frequent dosing is crucial. Using a computational and structure-based design strategy to guide lead optimization, a 5 µM virtual screening hit was transformed to a series of very potent nanomolar to picomolar catechol diethers. One representative, compound I, was shown to have nanomolar activity in HIV-1-infected T cells, potency on clinically relevant HIV-1 drug-resistant strains, lack of cytotoxicity and off-target effects, and excellent in vivo pharmacokinetic behavior. In this report, we show the feasibility of compound I as a late-stage preclinical candidate by establishing synergistic antiviral activity with existing HIV-1 drugs and clinical candidates and efficacy in HIV-1-infected humanized [human peripheral blood lymphocyte (Hu-PBL)] mice by completely suppressing viral loads and preventing human CD4+ T-cell loss. Moreover, a long-acting nanoformulation of compound I [compound I nanoparticle (compound I-NP)] in poly(lactide-coglycolide) (PLGA) was developed that shows sustained maintenance of plasma drug concentrations and drug efficacy for almost 3 weeks after a single dose.
Keywords: HIV-1; NNRTI; drug synergy; humanized mice; nanoparticle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Comment in
-
Future of nonnucleoside reverse transcriptase inhibitors.Proc Natl Acad Sci U S A. 2018 Jan 23;115(4):637-638. doi: 10.1073/pnas.1720975115. Epub 2018 Jan 11. Proc Natl Acad Sci U S A. 2018. PMID: 29326232 Free PMC article. No abstract available.
-
Reply to Pandey et al.: Understanding the efficacy of a potential antiretroviral drug candidate in humanized mouse model of HIV infection.Proc Natl Acad Sci U S A. 2018 Aug 28;115(35):E8114-E8115. doi: 10.1073/pnas.1810136115. Epub 2018 Aug 10. Proc Natl Acad Sci U S A. 2018. PMID: 30097540 Free PMC article. No abstract available.
-
Do the pharmacokinetic and pharmacodynamic graphs warrant additional explanation?Proc Natl Acad Sci U S A. 2018 Aug 28;115(35):E8113. doi: 10.1073/pnas.1808640115. Epub 2018 Aug 10. Proc Natl Acad Sci U S A. 2018. PMID: 30097541 Free PMC article. No abstract available.
References
-
- UNAIDS 2016 Global AIDS Update 2016. Available at http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update.... Accessed December 4, 2017.
-
- Schneider MF, et al. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984-2004. AIDS. 2005;19:2009–2018. - PubMed
-
- Wood E, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–554. - PubMed
-
- Palella FJ, Jr, et al. HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

