Dynamic force spectroscopy of synthetic oligorotaxane foldamers
- PMID: 29279384
- PMCID: PMC6156609
- DOI: 10.1073/pnas.1712790115
Dynamic force spectroscopy of synthetic oligorotaxane foldamers
Abstract
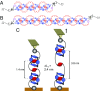
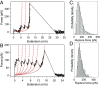
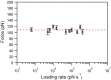
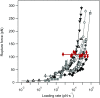
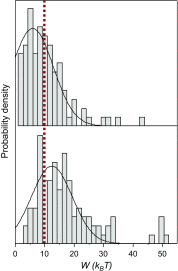
Wholly synthetic molecules involving both mechanical bonds and a folded secondary structure are one of the most promising architectures for the design of functional molecular machines with unprecedented properties. Here, we report dynamic single-molecule force spectroscopy experiments that explore the energetic details of donor-acceptor oligorotaxane foldamers, a class of molecular switches. The mechanical breaking of the donor-acceptor interactions responsible for the folded structure shows a high constant rupture force over a broad range of loading rates, covering three orders of magnitude. In comparison with dynamic force spectroscopy performed during the past 20 y on various (bio)molecules, the near-equilibrium regime of oligorotaxanes persists at much higher loading rates, at which biomolecules have reached their kinetic regime, illustrating the very fast dynamics and remarkable rebinding capabilities of the intramolecular donor-acceptor interactions. We focused on one single interaction at a time and probed the stochastic rupture and rebinding paths. Using the Crooks fluctuation theorem, we measured the mechanical work produced during the breaking and rebinding to determine a free-energy difference, ΔG, of 6 kcal·mol-1 between the two local conformations around a single bond.
Keywords: AFM; equilibrium dynamics; foldamers; molecular machines; single-molecule force spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kinbara K, Aida T. Toward intelligent molecular machines: Directed motions of biological and artificial molecules and assemblies. Chem Rev. 2005;105:1377–1400. - PubMed
-
- Berg HC. The rotary motor of bacterial flagella. Annu Rev Biochem. 2003;72:19–54. - PubMed
-
- Kay ER, Leigh DA, Zerbetto F. Synthetic molecular motors and mechanical machines. Angew Chem Int Ed Engl. 2007;46:72–191. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

