RSK2 drives cell motility by serine phosphorylation of LARG and activation of Rho GTPases
- PMID: 29279389
- PMCID: PMC5777029
- DOI: 10.1073/pnas.1708584115
RSK2 drives cell motility by serine phosphorylation of LARG and activation of Rho GTPases
Abstract
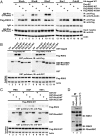
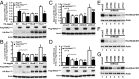
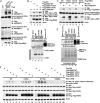
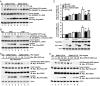
Directed migration is essential for cell motility in many processes, including development and cancer cell invasion. RSKs (p90 ribosomal S6 kinases) have emerged as central regulators of cell migration; however, the mechanisms mediating RSK-dependent motility remain incompletely understood. We have identified a unique signaling mechanism by which RSK2 promotes cell motility through leukemia-associated RhoGEF (LARG)-dependent Rho GTPase activation. RSK2 directly interacts with LARG and nucleotide-bound Rho isoforms, but not Rac1 or Cdc42. We further show that epidermal growth factor or FBS stimulation induces association of endogenous RSK2 with LARG and LARG with RhoA. In response to these stimuli, RSK2 phosphorylates LARG at Ser1288 and thereby activates RhoA. Phosphorylation of RSK2 at threonine 577 is essential for activation of LARG-RhoA. Moreover, RSK2-mediated motility signaling depends on RhoA and -B, but not RhoC. These results establish a unique RSK2-dependent LARG-RhoA signaling module as a central organizer of directed cell migration and invasion.
Keywords: ARHGEF12; LARG; RSK2; Rho GTPases; motility.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hauge C, Frödin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119:3021–3023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

