Diagnostics and therapy of vestibular schwannomas - an interdisciplinary challenge
- PMID: 29279723
- PMCID: PMC5738934
- DOI: 10.3205/cto000142
Diagnostics and therapy of vestibular schwannomas - an interdisciplinary challenge
Abstract
Vestibular schwannomas (VS) expand slowly in the internal auditory canal, in the cerebellopontine angle, inside the cochlear and the labyrinth. Larger tumors can displace and compress the brainstem. With an annual incidence of 1:100,000 vestibular schwannoma represent 6-7% of all intracranial tumors. In the cerebellopontine angle they are by far the most neoplasm with 90% of all lesions located in this region. Magnetic resonance imaging (MRI), audiometry, and vestibular diagnostics are the mainstays of the clinical workup for patients harboring tumors. The first part of this paper delivers an overview of tumor stages, the most common grading scales for facial nerve function and hearing as well as a short introduction to the examination of vestibular function. Upholding or improving quality of life is the central concern in counseling and treating a patient with vestibular schwannoma. Preservation of neuronal function is essential and the management options - watchful waiting, microsurgery and stereotactic radiation - should be custom-tailored to the individual situation of the patient. Continuing interdisciplinary exchange is important to monitor treatment quality and to improve treatment results. Recently, several articles and reviews have been published on the topic of vestibular schwannoma. On the occasion of the 88th annual meeting of the German Society of Oto-Rhino-Laryngology, Head and Neck surgery a special volume of the journal "HNO" will be printed. Hence this presentation has been designed to deviate from the traditional standard which commonly consists of a pure literature review. The current paper was conceptually woven around a series of interdisciplinary cases that outline examples for every stage of the disease that show characteristic results for management options to date. Systematic clinical decision pathways have been deduced from our experience and from results reported in the literature. These pathways are graphically outlined after the case presentations. Important criteria for decision making are size and growth rate of the tumor, hearing of the patient and the probability of total tumor resection with preservation of hearing and facial nerve function, age and comorbidity of the patient, best possible control of vertigo and tinnitus and last but not least the patient's preference and choice. In addition to this, the experience and the results of a given center with each treatment modality will figure in the decision making process. We will discuss findings that are reported in the literature regarding facial nerve function, hearing, vertigo, tinnitus, and headache and reflect on recent studies on their influence on the patient's quality of life. Vertigo plays an essential role in this framework since it is an independent predictor of quality of life and a patient's dependence on social welfare. Pathognomonic bilateral vestibular schwannomas that occur in patients suffering from neurofibromatosis typ-2 (NF2) differ from spontaneous unilateral tumors in their biologic behavior. Treatment of neurofibromatosis type-2 patients requires a multidisciplinary team, especially because of the multitude of separate intracranial and spinal lesions. Off-label chemotherapy with Bevacizumab can stabilize tumor size of vestibular schwannomas and even improve hearing over longer periods of time. Hearing rehabilitation in NF2 patients can be achieved with cochlear and auditory brainstem implants.
Keywords: acoustic neuroma; management; microsurgery; quality of life; radiosurgery; vestibular schwannoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






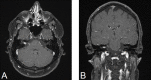



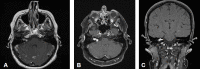


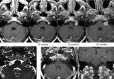
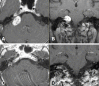
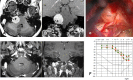
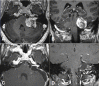
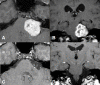

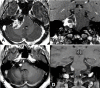


















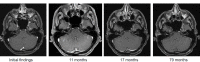
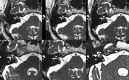




References
-
- Böcker W, Denk H, Heitz PU, Moch H. Pathologie. 4 ed. Münschen, Jena: Urban & Fischer Verlag/Elsevier GmbH; 2008.
-
- Probst R, Grevers G, Iro H. Hals-Nasen-Ohren-Heilkunde. 3 ed. Stuttgart: Thieme; 2008.
-
- Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988 May;45(5):575–578. - PubMed
-
- Baser ME, Friedman JM, Joe H, Shenton A, Wallace AJ, Ramsden RT, Evans DG. Empirical development of improved diagnostic criteria for neurofibromatosis 2. Genet Med. 2011 Jun;13(6):576–581. doi: 10.1097/GIM.0b013e318211faa9. Available from: http://dx.doi.org/10.1097/GIM.0b013e318211faa9. - DOI - DOI - PubMed
-
- Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V, Harris R. A clinical study of type 2 neurofibromatosis. Q J Med. 1992 Aug;84(304):603–618. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

