Implantable hearing devices
- PMID: 29279724
- PMCID: PMC5738935
- DOI: 10.3205/cto000145
Implantable hearing devices
Abstract
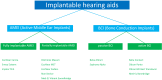
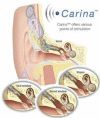
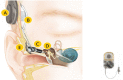
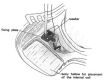
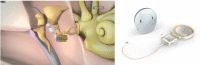
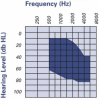
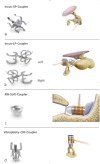
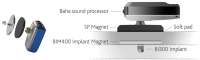
Combined hearing loss is an essential indication for implantable hearing systems. Depending on the bone conduction threshold, various options are available. Patients with mild sensorineural deafness usually benefit from transcutaneous bone conduction implants (BCI), while percutaneous BCI systems are recommended also for moderate hearing loss. For combined hearing losses with moderate and high-grade cochlear hearing loss, active middle ear implants are recommended. For patients with incompatibilities or middle ear surgery, implants are a valuable and proven addition to the therapeutic options.
Keywords: active middle ear implant; active percutaneous systems; active transcutaneous systems; bone conduction implants; fully implantable systems; hearing disorder; hearing loss; middle ear implant; partially implantable systems; passive systems.
Conflict of interest statement
The author received lecture fees and travel expenses from the company MED-EL in the past.
Figures

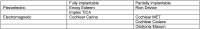











References
-
- Biha. Hörgeminderte und Hörgeräte-Versorgung in Deutschland im Jahr 2016. Statista - Das Statistik-Portal. 2016. [cited 2016 Sep 09]. Available from: http://de.statista.com/statistik/daten/studie/71443/umfrage/hoergeminder...
-
- Löhler J, Akcicek B, Wollenberg B, Schönweiler R. Umsetzung der neuen Qualitätssicherungsvereinbarung zur Hörgeräteversorgung im Praxisalltag: Teil 1 Neue Regeln der Hörgeräteverordnung. HNO. 2014;62:605–612. doi: 10.1007/s00106-014-2879-4. Available from: http://dx.doi.org/10.1007/s00106-014-2879-4. - DOI - PubMed
-
- Arbeitsgemeinschaft Deutschprachiger Audiologen und Neurootologen der DGHNO. AWMF-Leitlinie: Aktive, implantierbare Hörsysteme bei Hörstörungen - Registernummer: 17/073, Klasse: S1, Version: 04/2010. 2010. [cited 2016 Sep 09]. Available from: http://www.awmf.org/uploads/tx_szleitlinien/017-073l_S1_Aktive_implantie....
-
- Zenner HP. Innenohrschwerhörigkeit: Elektronische Hörimplantate zur operativen Behandlung. Dtsch Ärztebl. 2001;98:A169–A174.
-
- Colletti V, Soli SD, Carner M, Colletti L. Treatment of mixed hearing losses via implantation of a vibratory transducer on the round window. Int J Audiol. 2006 Oct;45(10):600–608. doi: 10.1080/14992020600840903. Available from: http://dx.doi.org/10.1080/14992020600840903. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

