The Expression of AQP5 and UTs in the Sweat Glands of Uremic Patients
- PMID: 29279852
- PMCID: PMC5723962
- DOI: 10.1155/2017/8629783
The Expression of AQP5 and UTs in the Sweat Glands of Uremic Patients
Abstract
Purpose: To research the distribution and quantitative changes of UT-A1, UT-B1, and AQP5 in uremic skin tissue.
Methods: 34 cases of uremic patients (UP) and 11 controls were recruited. Immunohistochemistry, immunofluorescence, RT-PCR, and Western Blot were used to identify the proteins in sweat glands.
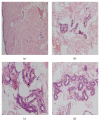
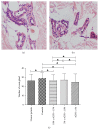
Results: AQP5, UT-A1, and UT-B1 were expressed and localized in human skin basal lines, skin sweat glands, and sweat ducts, both in UP and controls. Compared to controls, AQP5 mRNA abundance was significantly decreased in UP (P < 0.01), and, with the decrease of eGFR, the AQP5 expression was significantly decreased (P < 0.05). By contrast, UT-A1 and UT-B1 mRNA abundance was significantly increased in the skin of UP compared with the control (P < 0.01), and, with the decrease of eGFR, the AQP5 expression was significantly increased (P < 0.05). We found that the gene changes were coincident with the corresponding target proteins. The urea transporter subtypes, UT-A1 and UT-B1, were expressed in the skin basal cell layer and exocrine sweat glands. The abundance of UT-A1 and UT-B1 in uremic sweat glands was significantly increased in UP, while the expression of AQP5 was decreased.
Conclusion: Elimination of urea through the skin by producing sweat is a potential therapeutic strategy for renal failure patients.
Figures





Similar articles
-
[Expression of urea transporters in sweat gland tissue of normal subjects and uremic patients].Nan Fang Yi Ke Da Xue Xue Bao. 2013 Jul;33(7):951-5. Nan Fang Yi Ke Da Xue Xue Bao. 2013. PMID: 23895831 Chinese.
-
Functional requirement of aquaporin-5 in plasma membranes of sweat glands.Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):511-6. doi: 10.1073/pnas.012588099. Epub 2002 Jan 2. Proc Natl Acad Sci U S A. 2002. PMID: 11773623 Free PMC article.
-
Postnatal expression and denervation induced up-regulation of aquaporin-5 protein in rat sweat gland.Cell Tissue Res. 2007 Jul;329(1):25-33. doi: 10.1007/s00441-007-0399-1. Epub 2007 Mar 23. Cell Tissue Res. 2007. PMID: 17380350
-
New Findings on the Mechanism of Perspiration Including Aquaporin-5 Water Channel.Curr Probl Dermatol. 2016;51:11-21. doi: 10.1159/000446754. Epub 2016 Aug 30. Curr Probl Dermatol. 2016. PMID: 27584958 Review.
-
Renal urea transporters.Curr Opin Nephrol Hypertens. 2004 Sep;13(5):525-32. doi: 10.1097/00041552-200409000-00008. Curr Opin Nephrol Hypertens. 2004. PMID: 15300159 Review.
Cited by
-
Integrated Transcriptomic and Proteomic Analysis of Human Eccrine Sweat Glands Identifies Missing and Novel Proteins.Mol Cell Proteomics. 2019 Jul;18(7):1382-1395. doi: 10.1074/mcp.RA118.001101. Epub 2019 Apr 12. Mol Cell Proteomics. 2019. PMID: 30979791 Free PMC article.
-
Current State of SLC and ABC Transporters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases.Proteomes. 2021 May 16;9(2):23. doi: 10.3390/proteomes9020023. Proteomes. 2021. PMID: 34065737 Free PMC article. Review.
-
Physiological mechanisms determining eccrine sweat composition.Eur J Appl Physiol. 2020 Apr;120(4):719-752. doi: 10.1007/s00421-020-04323-7. Epub 2020 Mar 2. Eur J Appl Physiol. 2020. PMID: 32124007 Free PMC article. Review.
-
Physiology of sweat gland function: The roles of sweating and sweat composition in human health.Temperature (Austin). 2019 Jul 17;6(3):211-259. doi: 10.1080/23328940.2019.1632145. eCollection 2019. Temperature (Austin). 2019. PMID: 31608304 Free PMC article. Review.
-
A novel kinetic model estimating the urea concentration in plasma during non-invasive sweat-based monitoring in hemodialysis.Front Physiol. 2025 Mar 18;16:1547117. doi: 10.3389/fphys.2025.1547117. eCollection 2025. Front Physiol. 2025. PMID: 40171116 Free PMC article.
References
-
- Huang C. T., Chen M. L., Huang L. L., Mao I. F. Uric acid and urea in human sweat. The Chinese Journal of Physiology. 2002;45(3):109–115. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

